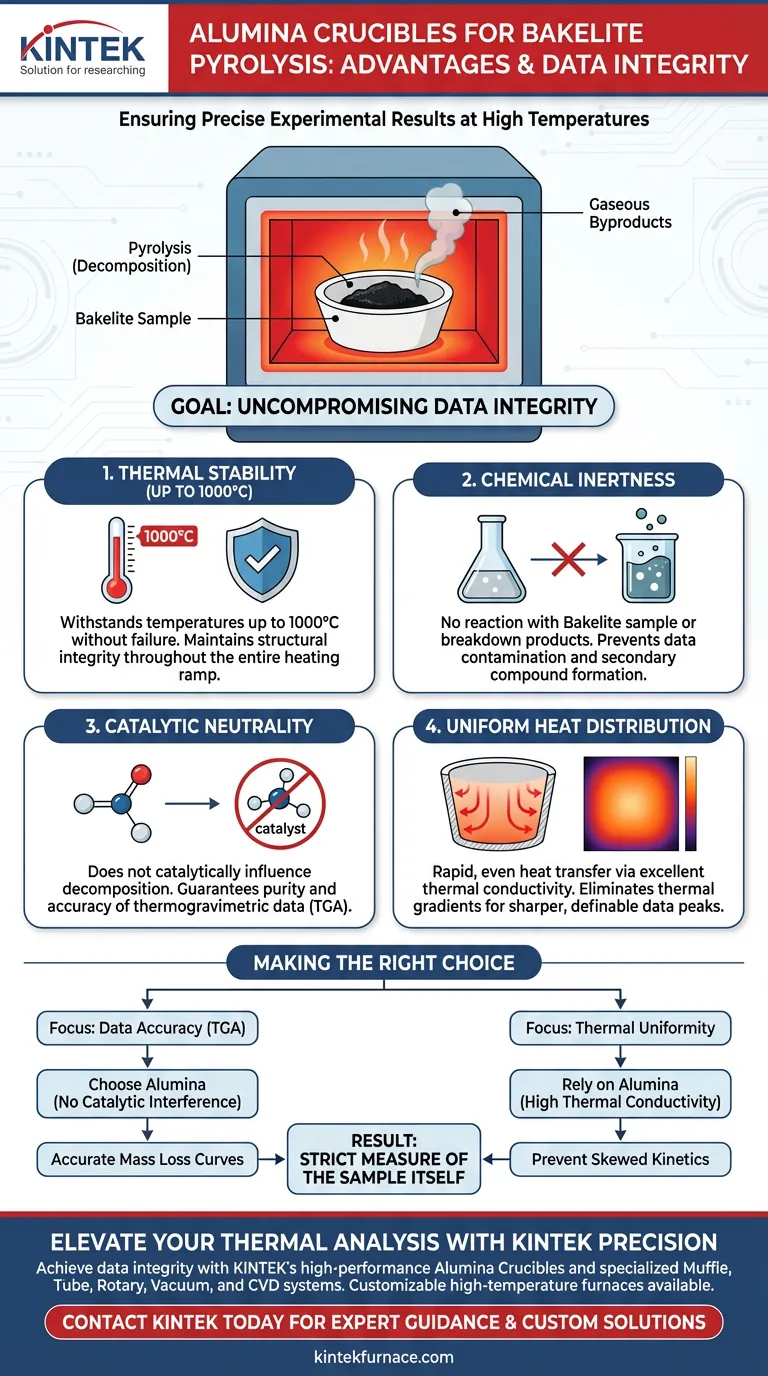
Alumina (Al2O3) crucibles are the superior choice for the high-temperature pyrolysis of Bakelite due to their unique combination of thermal stability and chemical neutrality. They allow for testing up to 1000°C without reacting with the sample, ensuring that the decomposition process remains uninfluenced by the container. Furthermore, their excellent thermal conductivity guarantees uniform heating, which is critical for obtaining precise experimental results.
The core advantage of using alumina is the preservation of data integrity. By effectively remaining "invisible" to the chemical process, alumina crucibles ensure that the thermogravimetric data you collect reflects the true properties of the Bakelite, free from catalytic interference or thermal gradients.

Ensuring Experimental Integrity
To understand why alumina is the standard for this application, it is necessary to look at how its physical properties directly support the rigorous demands of thermal analysis.
High-Temperature Stability
Pyrolysis requires subjecting samples to extreme heat to induce decomposition. Alumina crucibles offer exceptional thermal stability, allowing them to withstand testing temperatures up to 1000°C.
This high threshold ensures the crucible maintains its structural integrity throughout the entire heating ramp. You can confidently reach the decomposition point of Bakelite without risking crucible failure.
Chemical Inertness
In accurate thermal analysis, the container must never become part of the reaction. Alumina is chemically inert, meaning it will not react with the Bakelite sample even as the sample breaks down into reactive byproducts.
This isolation is vital. It prevents the formation of secondary compounds that could alter the mass loss profile or heat flow data.
Eliminating Catalytic Interference
Some crucible materials can inadvertently act as catalysts, accelerating or altering the decomposition pathway of a polymer.
Alumina does not catalytically influence the decomposition of Bakelite. This neutrality ensures the purity and accuracy of your thermogravimetric data, giving you a clear picture of the material's standalone behavior.
Uniform Heat Distribution
Accurate pyrolysis data relies on the entire sample experiencing the same temperature at the same time. Alumina possesses excellent thermal conductivity, which facilitates rapid and even heat transfer.
This prevents thermal gradients—"hot spots" or "cold spots"—within the sample. Uniform heating ensures that the decomposition occurs simultaneously throughout the material, leading to sharper, more definable data peaks.
Understanding the Constraints
While alumina is highly effective, it is essential to recognize the operational boundaries defined by its physical properties.
The Temperature Ceiling
While robust, the specific advantage cited applies to temperatures up to 1000°C.
If your experimental protocol requires temperatures significantly exceeding this threshold, or if you are pushing the upper limits of your furnace, you must verify that the specific grade of alumina used can maintain its inertness and stability without softening or reacting.
Making the Right Choice for Your Goal
Selecting the correct crucible is about matching the material properties to your specific data requirements.
- If your primary focus is Data Accuracy (TGA): Choose alumina to eliminate catalytic interference and ensure the mass loss curves represent only the Bakelite decomposition.
- If your primary focus is Thermal Uniformity: Rely on alumina’s high thermal conductivity to prevent thermal gradients that could skew reaction kinetics.
By using alumina crucibles, you effectively eliminate the variable of the container, leaving you with results that are strictly a measure of the sample itself.
Summary Table:
| Feature | Advantage for Bakelite Pyrolysis | Benefit to Researcher |
|---|---|---|
| Thermal Stability | Resists temperatures up to 1000°C | Ensures structural integrity during decomposition |
| Chemical Inertness | No reaction with sample or byproducts | Prevents data contamination and secondary reactions |
| Catalytic Neutrality | Does not alter decomposition pathways | Guarantees purity and accuracy of thermogravimetric data |
| Thermal Conductivity | Rapid and uniform heat transfer | Eliminates thermal gradients for sharper data peaks |
Elevate Your Thermal Analysis with KINTEK Precision
Achieve uncompromising data integrity in your pyrolysis experiments. Backed by expert R&D and manufacturing, KINTEK offers high-performance alumina crucibles alongside our specialized Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need standard lab equipment or a customizable high-temperature furnace tailored to your unique research needs, our team is ready to support your laboratory's success.
Ready to optimize your high-temperature processes? Contact KINTEK today for expert guidance and custom solutions!
Visual Guide
References
- Exploring the Thermal Degradation of Bakelite: Non-Isothermal Kinetic Modeling, Thermodynamic Insights, and Evolved Gas Analysis via Integrated In Situ TGA/MS and TGA/FT-IR Techniques. DOI: 10.3390/polym17162197
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of the laboratory furnace? Master Material Transformation with Precision Heating
- Why are high-purity alumina crucibles utilized for CsV3Sb5 crystal growth? Ensure Purity in Self-Flux Synthesis
- Why is the use of high-purity alumina crucibles essential for the synthesis of Ni3In2Se2? | Precision Material Purity
- What are the primary uses of quartz tubes in laboratory settings? Essential for High-Temperature Material Processing
- What is the primary function of a vacuum-sealed quartz tube in MnBi2Te4 growth? Ensure High-Purity Crystal Synthesis
- What is the maximum pressure achievable by the circulating water vacuum pump? Discover Its Vacuum Limits
- What role does a Molybdenum Boat play in ZTO thin film deposition? Master Thermal Evaporation Success
- Where are water circulating vacuum pumps commonly used? Essential for Lab and Industrial Vapor Handling



















