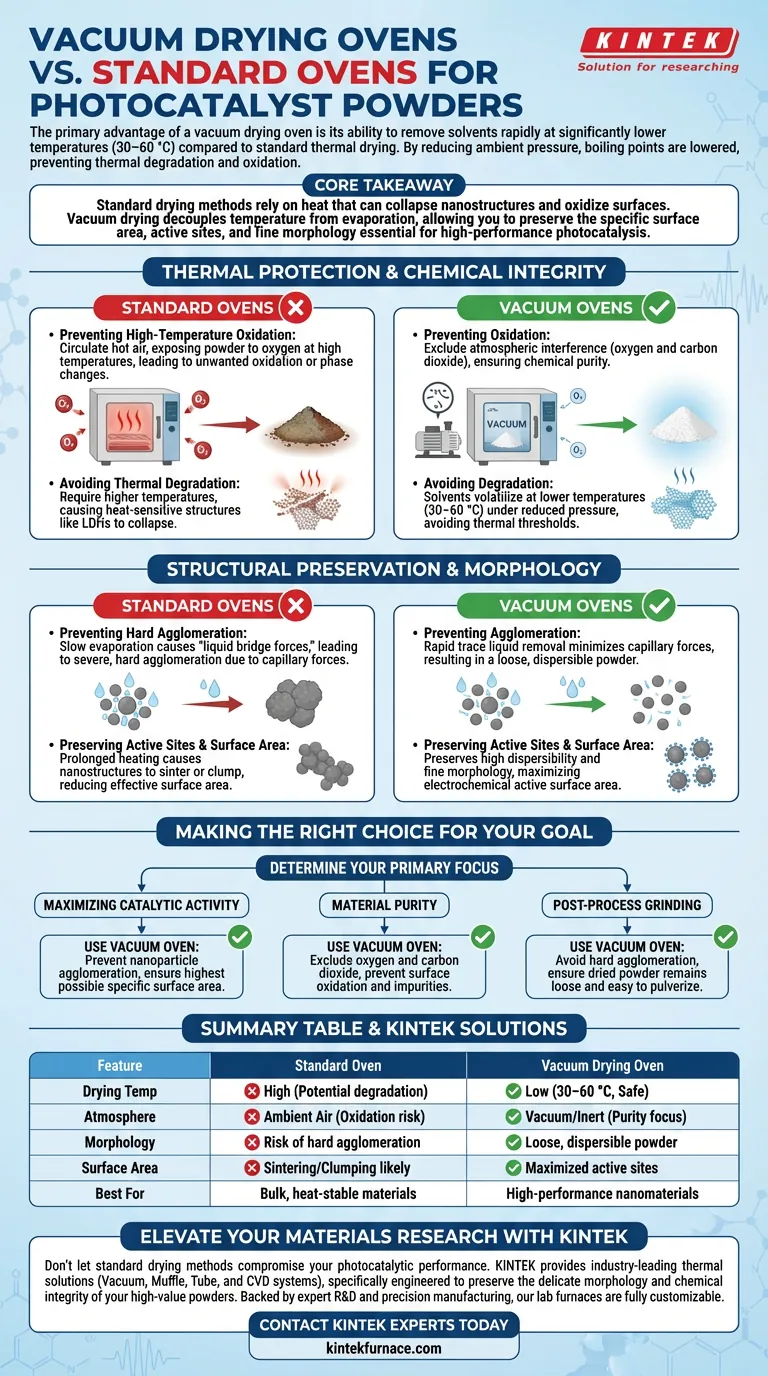
The primary advantage of a vacuum drying oven is its ability to remove solvents rapidly at significantly lower temperatures compared to standard thermal drying. By reducing the ambient pressure, you lower the boiling point of moisture and residual solvents (like ethanol), allowing for effective drying at temperatures as low as 30–60 °C. This prevents the thermal degradation and oxidation that often compromise the quality of photocatalyst powders in standard ovens.
Core Takeaway Standard drying methods rely on heat that can collapse nanostructures and oxidize surfaces. Vacuum drying decouples temperature from evaporation, allowing you to preserve the specific surface area, active sites, and fine morphology essential for high-performance photocatalysis.

Thermal Protection and Chemical Integrity
The most immediate benefit of vacuum drying is the preservation of the material's chemical composition. Standard ovens typically require higher temperatures to drive off solvents, which introduces significant risks to delicate catalysts.
Preventing High-Temperature Oxidation
Standard ovens circulate hot air, which exposes the powder to oxygen at high temperatures. This often leads to unwanted oxidation reactions or phase changes.
A vacuum oven operates by excluding atmospheric interference (oxygen and carbon dioxide). This oxygen-free environment is critical for materials prone to side reactions, ensuring the chemical purity of the active material surface is maintained.
Avoiding Thermal Degradation
Many photocatalyst precursors or composites, such as layered double hydroxides (LDHs), are heat-sensitive. High heat can cause these structures to collapse.
By lowering the system pressure, solvents volatilize at much lower temperatures (e.g., 60 °C or even 30 °C). This allows the material to dry completely without reaching the thermal threshold that would degrade its structure.
Structural Preservation and Morphology
Beyond chemical purity, the physical architecture of a photocatalyst—its shape and porosity—defines its efficiency. Vacuum drying is superior for maintaining these physical traits.
Preventing Hard Agglomeration
In standard drying, as liquid evaporates slowly, "liquid bridge forces" can pull particles together. This creates capillary forces that result in severe, hard agglomeration.
Vacuum drying facilitates the rapid removal of trace liquids from particle pores. This speed and mechanism minimize capillary forces, preventing the formation of hard clumps. The result is a loose, dispersible powder that is easier to grind and process.
Preserving Active Sites and Surface Area
The performance of a photocatalyst is directly tied to its specific surface area and the availability of active sites (such as platinum nanoparticles or MnMgPO4 particles).
Prolonged heating in a standard oven can cause these nanostructures to sinter or clump together, reducing their effective surface area. Vacuum drying preserves the high dispersibility of nanoparticles and the fine morphology of the composite, maximizing the electrochemical active surface area.
Understanding the Trade-offs
While vacuum drying offers superior quality for nanomaterials, it is important to apply it where it adds the most value.
Equipment Complexity vs. Necessity
Vacuum drying adds complexity to the process compared to a simple convection oven. It requires a vacuum pump and a sealable chamber, which introduces maintenance requirements for seals and pump oil.
Batch Processing Limitations
Vacuum ovens are typically batch-process devices. If your workflow requires continuous, high-throughput drying of bulk materials where surface area is not critical, a standard oven may be more efficient. Vacuum drying is best reserved for high-value powders where morphology and surface chemistry are non-negotiable.
Making the Right Choice for Your Goal
To determine if vacuum drying is necessary for your specific project, consider your performance metrics:
- If your primary focus is Maximizing Catalytic Activity: Use a vacuum oven to prevent nanoparticle agglomeration and ensure the highest possible specific surface area.
- If your primary focus is Material Purity: Use a vacuum oven to exclude oxygen and carbon dioxide, preventing surface oxidation and impurity formation (such as carbonates).
- If your primary focus is Post-Process Grinding: Use a vacuum oven to avoid hard agglomeration, ensuring the dried powder remains loose and easy to pulverize.
For high-performance photocatalysts, the vacuum drying oven is not just a drying tool; it is a synthesis control step that locks in the nanostructure you worked hard to create.
Summary Table:
| Feature | Standard Oven | Vacuum Drying Oven |
|---|---|---|
| Drying Temp | High (Potential degradation) | Low (30–60 °C, Safe) |
| Atmosphere | Ambient Air (Oxidation risk) | Vacuum/Inert (Purity focus) |
| Morphology | Risk of hard agglomeration | Loose, dispersible powder |
| Surface Area | Sintering/Clumping likely | Maximized active sites |
| Best For | Bulk, heat-stable materials | High-performance nanomaterials |
Elevate Your Materials Research with KINTEK
Don't let standard drying methods compromise your photocatalytic performance. KINTEK provides industry-leading thermal solutions, including Vacuum, Muffle, Tube, and CVD systems, specifically engineered to preserve the delicate morphology and chemical integrity of your high-value powders.
Backed by expert R&D and precision manufacturing, our lab furnaces are fully customizable to meet your unique experimental needs. Ensure your catalysts maintain maximum surface area and purity with the right technology.
Contact KINTEK Experts Today to find the perfect drying solution for your lab!
Visual Guide
References
- Construction of a 1D/0D/2D BiFeO <sub>3</sub> /Ag/g-C <sub>3</sub> N <sub>4</sub> Z-scheme heterojunction for enhanced visible light photocatalysis of methylene blue. DOI: 10.1039/d5ra04825g
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why is a vacuum annealing furnace required for Cu2Co1-xNaxSnS4 films? Optimize Your Thin Film Crystallization
- Why is a laboratory vacuum oven necessary for dehydrating chloride salt media? Ensure High-Purity TiH2 Preparation
- What role do vacuum furnaces play in the semiconductor industry? Essential for High-Purity Processing and Yield
- What factors influence the design and selection of heating elements in vacuum furnaces? Optimize for Temperature, Purity, and Cost
- What are the applications of high-temperature vacuum sintering furnaces? Essential for Aerospace, Electronics, and Medical Materials
- How does a Vacuum Drying Oven contribute to solid-state electrolyte films? Enhance Film Density and Purity
- How does resistance heating work in vacuum furnace elements? Master Precise Heat Control for Your Lab
- How does the vacuum environment benefit material processing? Achieve Superior Purity and Control



















