Beyond its primary function of accelerating the chemical reaction, a Ni/Al2O3 catalyst placed in an induction heating field serves a critical auxiliary role as a secondary, in-situ heat source. Specifically, the metallic nickel particles within the catalyst interact with the electromagnetic field to generate localized microscopic heat.
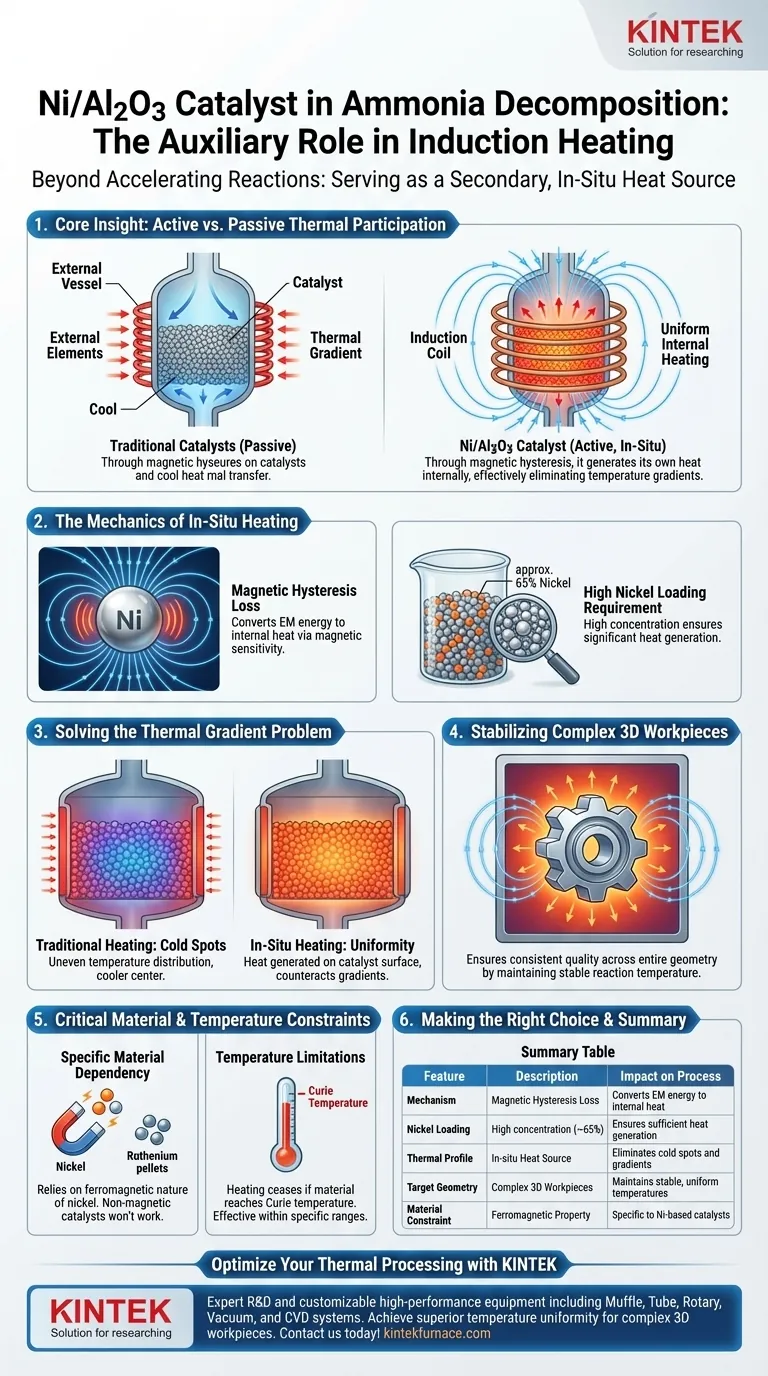
Core Insight While most catalysts are passive thermal recipients, a high-loading Ni/Al2O3 catalyst in an induction field becomes an active thermal participant. Through magnetic hysteresis, it generates its own heat internally, effectively eliminating temperature gradients that typically plague external heating methods.
The Mechanics of In-Situ Heating
Magnetic Heat Generation
The auxiliary heating effect is driven by the magnetic sensitivity of the nickel particles. When exposed to the alternating electromagnetic field of the induction system, these particles undergo hysteresis loss.
This process converts magnetic energy directly into thermal energy at a microscopic level. It transforms the catalyst bed from a static chemical medium into an active heating element.
High Loading Requirement
To achieve this auxiliary heating effect, standard catalyst compositions are often insufficient. The process specifically utilizes a high nickel loading (approximately 65%).
This high concentration of metallic nickel ensures there is enough magnetic material present to generate significant heat, supplementing the primary heating of the system.
Solving the Thermal Gradient Problem
Eliminating Cold Spots
In traditional reactor designs, heat is applied externally, often leading to uneven temperature distributions where the center of the bed is cooler than the walls.
Because the Ni/Al2O3 catalyst generates heat from within the bed itself, it counteracts these thermal gradients. The heat is produced exactly where the reaction occurs—on the catalyst surface.
Stabilizing 3D Workpieces
This internal heating mechanism is particularly beneficial for processing complex, 3D workpieces.
By providing a secondary source of heat that permeates the catalyst bed, the system assists these workpieces in maintaining a stable and uniform reaction temperature, ensuring consistent quality across the entire geometry of the part.
Critical Material Constraints
Specific Material Dependency
It is crucial to recognize that this auxiliary heating effect is not a property of all ammonia decomposition catalysts. It relies strictly on the ferromagnetic nature of nickel.
Catalysts based on non-magnetic metals (such as Ruthenium) or those with very low nickel loading will not exhibit this hysteresis heating effect.
Temperature Limitations
While the primary reference focuses on the heating benefit, engineers must remember that ferromagnetic heating via hysteresis generally ceases if the material reaches its Curie temperature.
Therefore, this auxiliary role is most effective within specific temperature ranges where the nickel remains magnetically active.
Making the Right Choice for Your Process
If you are designing an induction-based ammonia decomposition system, consider how the catalyst choice impacts your thermal management:
- If your primary focus is Temperature Uniformity: Select a catalyst with high nickel loading (~65%) to leverage the in-situ heating effect and eliminate gradients.
- If your primary focus is Processing Complex Geometries: Utilize this catalyst-heating strategy to ensure 3D workpieces maintain stable temperatures during the reaction.
By treating the catalyst as both a chemical accelerator and a thermal generator, you achieve a more efficient and uniform decomposition process.
Summary Table:
| Feature | Description | Impact on Process |
|---|---|---|
| Mechanism | Magnetic Hysteresis Loss | Converts EM energy to internal heat |
| Nickel Loading | High concentration (~65%) | Ensures sufficient heat generation |
| Thermal Profile | In-situ Heat Source | Eliminates cold spots and gradients |
| Target Geometry | Complex 3D Workpieces | Maintains stable, uniform temperatures |
| Material Constraint | Ferromagnetic Property | Specific to Ni-based catalysts |
Optimize Your Thermal Processing with KINTEK
Is your ammonia decomposition process suffering from uneven heating or inefficient reactions? Backed by expert R&D and manufacturing, KINTEK provides the advanced heating solutions you need. We offer a full range of high-performance equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable for your unique lab or industrial requirements.
Our specialized knowledge in induction heating and catalyst integration helps you achieve superior temperature uniformity for even the most complex 3D workpieces. Contact us today to discuss your custom furnace needs and see how our technical expertise can drive your research and production success.
Visual Guide
References
- Débora de Figueiredo Luiz, Jurriaan Boon. Use of a 3D Workpiece to Inductively Heat an Ammonia Cracking Reactor. DOI: 10.3390/suschem6040043
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- What is the role of a vacuum induction furnace in MRDO preparation? Enabling Rare Earth Magnet Recycling
- What is the role of a Vacuum Induction Melting Furnace in chromium-steel prep? Secure Purity & Composition Control
- What are the advantages of induction heating over other melting methods? Unlock Speed, Purity & Control
- What industries and applications use Vacuum Induction Melting Furnaces? Essential for High-Purity Materials
- What is an IGBT induction furnace? Unlock Modern Efficiency in Metal Melting
- What is the function of a vacuum induction melting furnace for AlCoCrFeNi2.1? Mastering High-Entropy Alloy Production
- How does an IGBT Vacuum Induction Melting Furnace operate? Achieve Ultra-High-Purity Metal Melting
- What is the role of a Vacuum Arc Melting Furnace in HEA prep? Achieve Perfect Alloy Homogeneity



















