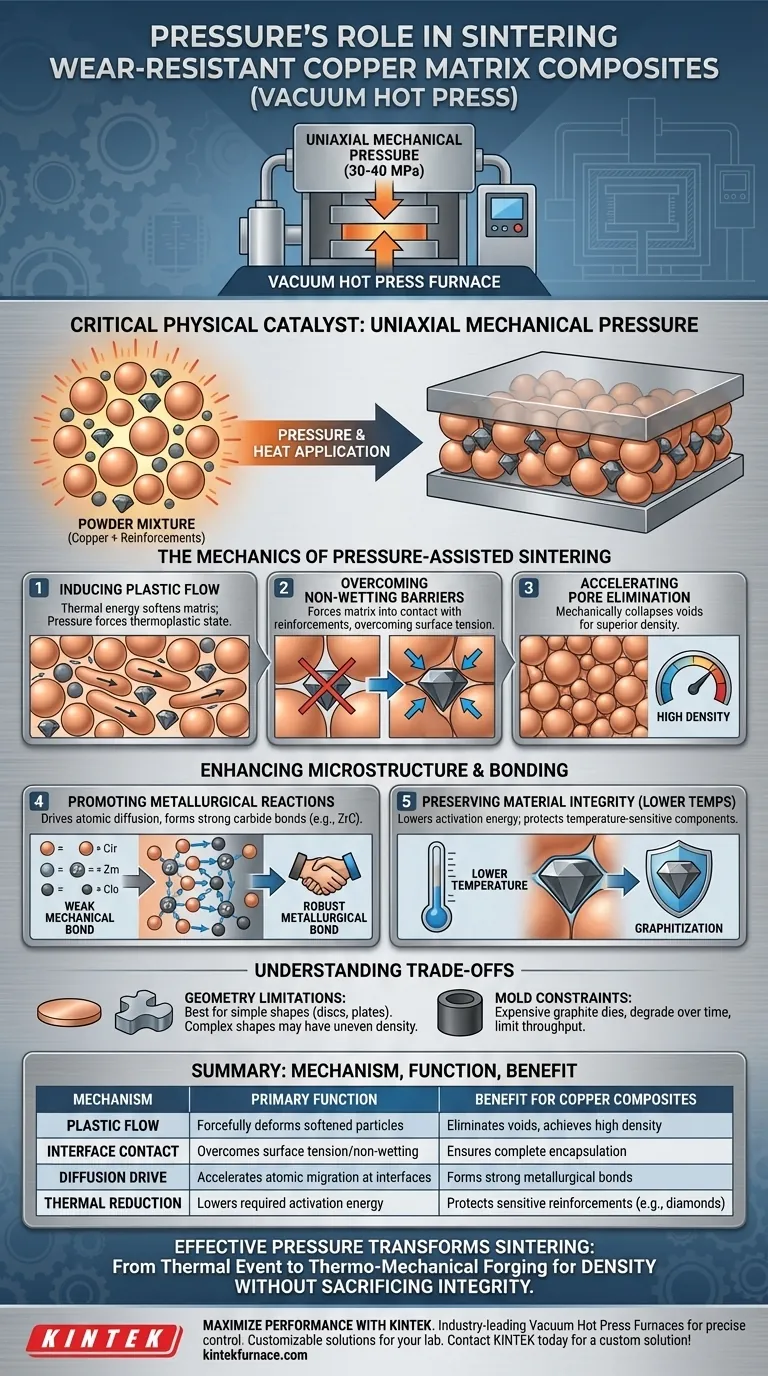
Uniaxial mechanical pressure acts as a critical physical catalyst that allows densification to occur where thermal energy alone would fail. By applying significant force (typically 30–40 MPa) directly to the powder mixture during the heating phase, the furnace induces plastic flow and particle rearrangement, physically crushing voids and forcing the copper matrix to conform around rigid wear-resistant particles.
Core Takeaway The application of pressure serves two simultaneous functions: it mechanically forces densification to overcome the natural non-wetting behavior of copper composites, and it allows sintering to occur at lower temperatures. This duality preserves the integrity of temperature-sensitive components (like diamonds) while ensuring a structurally sound, void-free matrix.

The Mechanics of Pressure-Assisted Sintering
Inducing Plastic Flow
Thermal energy softens the copper matrix, but pressure is required to move it. The application of uniaxial pressure forces the metal particles into a thermoplastic state. This causes the particles to slide past one another and deform, filling the interstitial spaces that would otherwise remain as pores.
Overcoming Non-Wetting Barriers
Copper naturally resists bonding with certain carbon-based materials, such as graphite or diamond. High mechanical pressure overcomes this surface tension and non-wetting issue by physically forcing the matrix into contact with the reinforcement phase. This ensures complete encapsulation of the wear-resistant particles, which is impossible with pressureless sintering.
Accelerating Pore Elimination
In standard sintering, pores close slowly via diffusion; in hot pressing, they are mechanically collapsed. The external force forcibly eliminates voids between particles, significantly increasing the final density of the composite. This results in a bulk material with superior mechanical properties and fewer structural defects.
Enhancing Microstructure and Bonding
Promoting Metallurgical Reactions
Pressure does not just pack particles; it drives atomic diffusion. The mechanical energy helps drive alloying elements (such as Zirconium) to the interface, triggering reactions that form carbides (e.g., Zirconium Carbide). This transitions the composite from a weak mechanical bond to a robust metallurgical bond.
Preserving Material Integrity via Lower Temperatures
Pressure lowers the activation energy required for densification. This allows the process to achieve high density at lower temperatures or significantly shorter holding times. Reducing thermal exposure is critical for preventing the graphitization of diamonds and stopping excessive grain growth in the copper matrix.
Understanding the Trade-offs
Geometry Limitations
The pressure is applied uniaxially (from top and bottom). This makes the process excellent for simple shapes like discs or plates but unsuitable for complex, non-symmetrical geometries which may experience uneven density.
Mold Constraints
The process relies on graphite dies to transmit pressure at high temperatures. These molds are expensive, degrade over time, and limit the throughput of the manufacturing process compared to continuous sintering methods.
Making the Right Choice for Your Goal
- If your primary focus is preserving diamond hardness: Prioritize higher pressure to allow for lower sintering temperatures, keeping the process below the graphitization threshold (approx. 1000°C).
- If your primary focus is interfacial bond strength: Ensure the pressure is maintained during the peak temperature hold to drive the diffusion of active elements (like Zr or Ti) for carbide formation.
- If your primary focus is maximum density: Utilize the pressure specifically to overcome the non-wetting characteristics between the copper matrix and the graphite/ceramic reinforcements.
Effective use of pressure transforms the sintering process from a thermal event into a thermo-mechanical forging, ensuring density without sacrificing material integrity.
Summary Table:
| Mechanism | Primary Function | Benefit for Copper Composites |
|---|---|---|
| Plastic Flow | Forcefully deforms softened metal particles | Eliminates voids and achieves near-theoretical density |
| Interface Contact | Overcomes surface tension/non-wetting | Ensures complete encapsulation of wear-resistant particles |
| Diffusion Drive | Accelerates atomic migration at interfaces | Forms strong metallurgical bonds via carbide formation |
| Thermal Reduction | Lowers required activation energy | Protects temperature-sensitive reinforcements like diamonds |
Maximize Your Material Performance with KINTEK
Precision engineering demands precise control over temperature and pressure. KINTEK provides industry-leading Vacuum Hot Press Furnaces, Muffle, Tube, and CVD systems designed to meet the rigorous requirements of advanced material synthesis.
Whether you are developing wear-resistant copper matrix composites or high-performance alloys, our expert R&D and manufacturing teams offer customizable lab high-temp furnaces tailored to your unique specifications. Ensure superior density and structural integrity for your projects today.
Ready to elevate your lab's capabilities? Contact KINTEK today for a custom solution!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How do you maintain a heat press machine? A Proactive Guide to Consistent Prints & Longevity
- What are the primary technical advantages of using a Spark Plasma Sintering (SPS) system? Achieve Superior Sintering
- Why are graphite molds critical for Cu/rGO vacuum hot pressing? Enhance Precision and Densification
- How do temperature, pressure, and vacuum affect material bonding and microstructure in vacuum hot pressing? Optimize for High-Performance Materials
- Why must vacuum hot press pressure be adjusted for SiC fiber spacing? Optimize Titanium Matrix Composites
- What are the primary applications of vacuum press technology? Achieve Superior Material Bonding and Shaping
- How does the pressurization system of a vacuum hot press affect SiC/TB8 composites? Optimize Matrix Densification
- Why is an industrial hot press critical for lunar ceramic components? Achieve Maximum Density and Impact Resistance



















