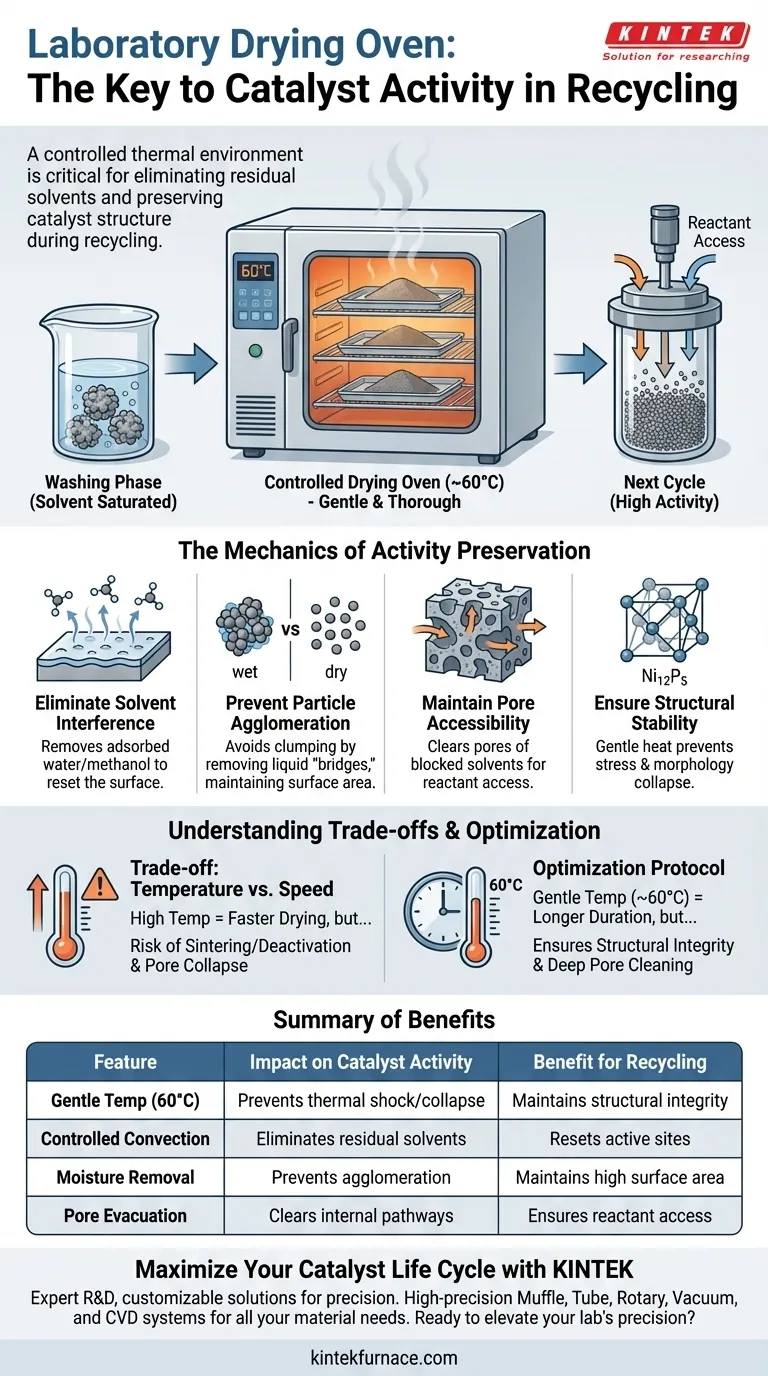
A laboratory drying oven acts as a critical stabilization tool in catalyst recycling by providing a controlled thermal environment to eliminate residual solvents without damaging the material. By maintaining a gentle temperature, typically around 60°C, it thoroughly removes moisture or methanol adsorbed on the catalyst surface following the washing phase.
The drying process is not merely about removing liquid; it is a structural preservation step. By eliminating solvent residues gently, you prevent the physical agglomeration of particles and the blockage of pores, ensuring the catalyst retains the specific surface area required for high activity in subsequent cycles.

The Mechanics of Activity Preservation
Eliminating Solvent Interference
After a catalytic cycle, the material undergoes a washing phase to remove reactants and byproducts. This leaves the catalyst saturated with solvents, such as water or methanol.
A drying oven set to approximately 60°C provides the necessary energy to drive off these adsorbates. This step is essential to "reset" the catalyst surface for the next use.
Preventing Particle Agglomeration
Wet catalyst particles have a natural tendency to clump together due to capillary forces and surface tension. If these clusters act as a single unit, the effective surface area drops significantly.
The drying process removes the liquid "bridge" between particles. This prevents permanent agglomeration, ensuring the powder remains fine and discrete.
Maintaining Pore Accessibility
Catalytic activity often depends on the material's porosity. Residual solvents trapped inside these pores can physically block reactants from reaching active sites.
By thoroughly drying the material, the oven ensures that the internal pore structure remains open. This creates an unobstructed pathway for reactants in the next experimental cycle.
Ensuring Structural Stability
Materials like Ni12P5 catalysts require specific handling to maintain their lattice structure. Rapid or harsh drying can induce stress on the material.
The controlled, gentle heat of the laboratory oven avoids these stresses. This preserves the structural integrity of the catalyst, allowing it to maintain performance stability over multiple recycling loops.
Understanding the Trade-offs
Temperature Sensitivity vs. Drying Speed
There is often a temptation to increase temperature to speed up the recycling process. However, this introduces a significant risk of morphology collapse.
High temperatures can cause the material structure to sinter or surface functional groups to deactivate. Sticking to a lower temperature (e.g., 60°C) protects the material but requires a longer duration to achieve complete dryness.
Atmosphere Control
Standard drying ovens rely on convection, which is effective for surface moisture but may be slower for deep pores.
While a standard oven prevents agglomeration, it lacks the pressure reduction of a vacuum oven. Consequently, standard drying may require extended timeframes to guarantee that deep-pore solvents are fully evacuated.
Optimizing Your Drying Protocol
To maximize the longevity and activity of your recycled catalysts, consider the specific requirements of your material and timeline.
- If your primary focus is Structural Integrity: Stick to a gentle drying temperature (around 60°C) to prevent thermal shock, pore collapse, or agglomeration of sensitive catalysts like Ni12P5.
- If your primary focus is Deep Pore Cleaning: Ensure the drying duration is sufficient to fully evacuate solvents from internal pores, preventing blockage that inhibits activity.
The laboratory drying oven effectively bridges the gap between experimental cycles, resetting the physical state of the catalyst to ensure consistent, repeatable data.
Summary Table:
| Feature | Impact on Catalyst Activity | Benefit for Recycling |
|---|---|---|
| Gentle Temperature (60°C) | Prevents thermal shock and morphology collapse | Maintains structural integrity over multiple cycles |
| Controlled Convection | Eliminates residual solvents (water/methanol) | Resets active sites for subsequent reactions |
| Moisture Removal | Prevents particle agglomeration | Maintains high specific surface area |
| Pore Evacuation | Clears internal pathways | Ensures reactant access to internal active sites |
Maximize Your Catalyst Life Cycle with KINTEK
Don't let improper drying compromise your experimental results. At KINTEK, we understand that structural preservation is key to catalyst longevity. Backed by expert R&D and manufacturing, we offer a wide range of laboratory equipment including high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique material needs.
Whether you are refining a delicate Ni12P5 catalyst or managing high-volume material recycling, our high-temperature solutions provide the stable, controlled environments required for peak performance.
Ready to elevate your lab's precision? Contact us today to find your custom furnace solution!
Visual Guide
References
- Omkar V. Vani, Anil M. Palve. Solar‐Powered Remediation of Carcinogenic Chromium(VI) and Methylene Blue Using Ferromagnetic Ni<sub>12</sub>P<sub>5</sub> and Porous Ni<sub>12</sub>P<sub>5</sub>‐rGO Nanostructures. DOI: 10.1002/metm.70010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why must the reaction containers be sealed within a fused quartz tube? Protect Your Crystal Growth Integrity
- What is the sucking rate for a single tap on the water circulating vacuum pump? Get Key Specs for Your Lab
- Alumina vs. Platinum Crucibles for Lithium Titanate (LTO) Synthesis: Which is Right for You?
- What roles do high-purity graphite molds play in SPS? Unlock the Secret to Superior Spark Plasma Sintering
- What are the main types of laboratory furnaces? Find Your Perfect High-Temperature Solution
- Where are water circulating vacuum pumps commonly used? Essential for Lab and Industrial Vapor Handling
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- What is the purpose of using a PID controller to drive a heating furnace? Master Thermal Kinetics Precision


















