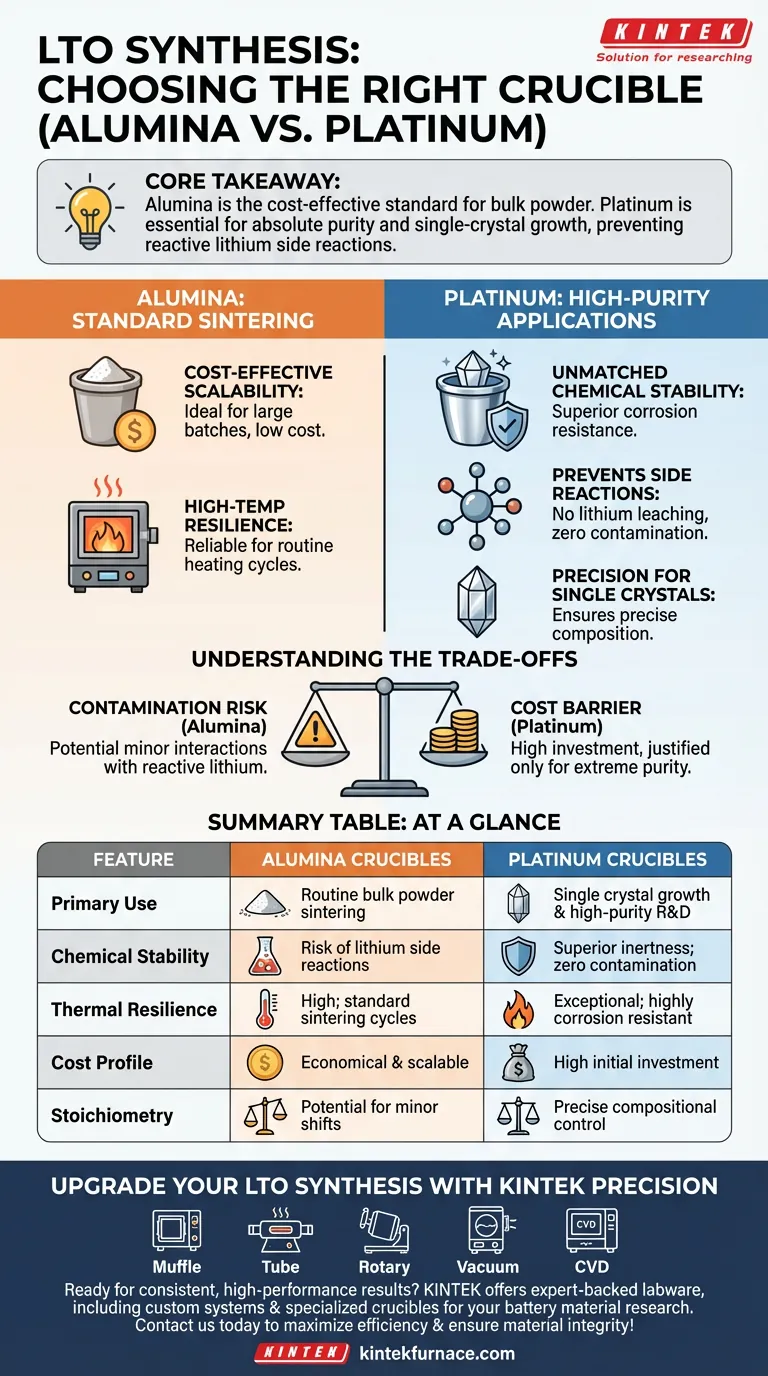
The choice between alumina and platinum crucibles for Lithium Titanate (LTO) synthesis depends primarily on the required purity of your final product and the specific synthesis method employed. Alumina is the industry standard for general solid-state sintering due to its balance of thermal resilience and low cost, while platinum is reserved for applications demanding absolute chemical inertness, such as single crystal growth.
Core Takeaway While alumina is sufficient for routine bulk powder synthesis, it cannot match the chemical stability of platinum in aggressive environments. If your process involves extended high temperatures or requires precise stoichiometry without lithium loss, platinum is the only option to prevent side reactions with the crucible walls.

The Role of Alumina: Standard Sintering
Cost-Effective Scalability
For most standard solid-state synthesis procedures, alumina crucibles are the preferred choice. They offer a significant economic advantage, making them ideal for large batches or iterative experiments where consumable costs must be minimized.
High-Temperature Resilience
Alumina provides excellent resistance to the high temperatures required for sintering LTO. It maintains structural integrity during standard heating cycles, making it a reliable workhorse for routine powder preparation.
The Role of Platinum: High-Purity Applications
Unmatched Chemical Stability
When the chemical integrity of the sample is paramount, platinum crucibles are required. Unlike alumina, platinum possesses superior corrosion resistance.
Preventing Side Reactions
Lithium salts are highly reactive at elevated temperatures. Platinum ensures that no side reactions occur between the lithium precursors and the crucible material. This prevents the leaching of crucible elements into the LTO sample.
Precision for Single Crystals
For the preparation of lithium titanate single crystals, platinum is non-negotiable. The growth of single crystals requires precise compositional control, which can only be achieved if the crucible remains chemically inert throughout the process.
Understanding the Trade-offs
The Contamination Risk
The primary drawback of using alumina is the potential for minor chemical interactions. In high-precision contexts, reactive lithium can attack the alumina wall, potentially altering the stoichiometry of your final product.
The Cost Barrier
Platinum eliminates contamination risks but introduces a steep barrier to entry regarding cost. Its use is typically justified only when the specific material properties (such as single-crystal structure) or purity levels (ppm-level analysis) demand it.
Making the Right Choice for Your Goal
To select the appropriate crucible for your specific LTO project:
- If your primary focus is standard powder synthesis: Choose alumina to maximize cost-efficiency while maintaining sufficient thermal resistance for sintering.
- If your primary focus is single crystal growth or high purity: Choose platinum to guarantee zero side reactions with lithium salts and ensure precise compositional control.
Select the material that matches the precision required by your specific application.
Summary Table:
| Feature | Alumina Crucibles | Platinum Crucibles |
|---|---|---|
| Primary Use | Routine bulk powder sintering | Single crystal growth & high-purity R&D |
| Chemical Stability | Risk of lithium side reactions | Superior inertness; zero contamination |
| Thermal Resilience | High; standard sintering cycles | Exceptional; highly corrosion resistant |
| Cost Profile | Economical & scalable | High initial investment |
| Stoichiometry | Potential for minor shifts | Precise compositional control |
Upgrade Your LTO Synthesis with KINTEK Precision
Ready to achieve consistent, high-purity lithium titanate results? Whether you need the cost-effective scalability of alumina or the unmatched chemical stability of platinum, KINTEK provides the high-performance labware your research demands.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized crucibles tailored for high-temperature synthesis. All our systems are fully customizable to meet the unique needs of your battery material research.
Maximize your lab's efficiency and ensure material integrity—Contact KINTEK today to discuss your custom furnace and crucible requirements.
Visual Guide
References
- C. Julien, A. Mauger. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. DOI: 10.3390/mi15030310
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What customization options are available for laboratory furnaces? Tailor Your Furnace for Precise Thermal Control
- Can alumina ceramic furnace tubes be customized for specific applications? Enhance Your High-Temperature Processes
- Can alumina ceramic furnace tubes be reused? Maximize Cost Savings and Safety
- How does an Aluminum Oxide Crucible ensure MXene purity? Key Role of LSS Etching Protection
- What is the role of providing a uniform heating environment? Achieve Perfect Deep Eutectic Solvent Formation
- What are the advantages of using an infrared thermograph over traditional thermocouples in Plasma Flash Sintering (PFS)?
- Why is a quartz boat required during APCVD for MoO2? Ensure High-Purity Single-Crystal Nanobelt Synthesis
- Why use sealed quartz tubes & vacuum for Mg-Zn/Mg-Cd alloy prep? Ensure Compositional Purity



















