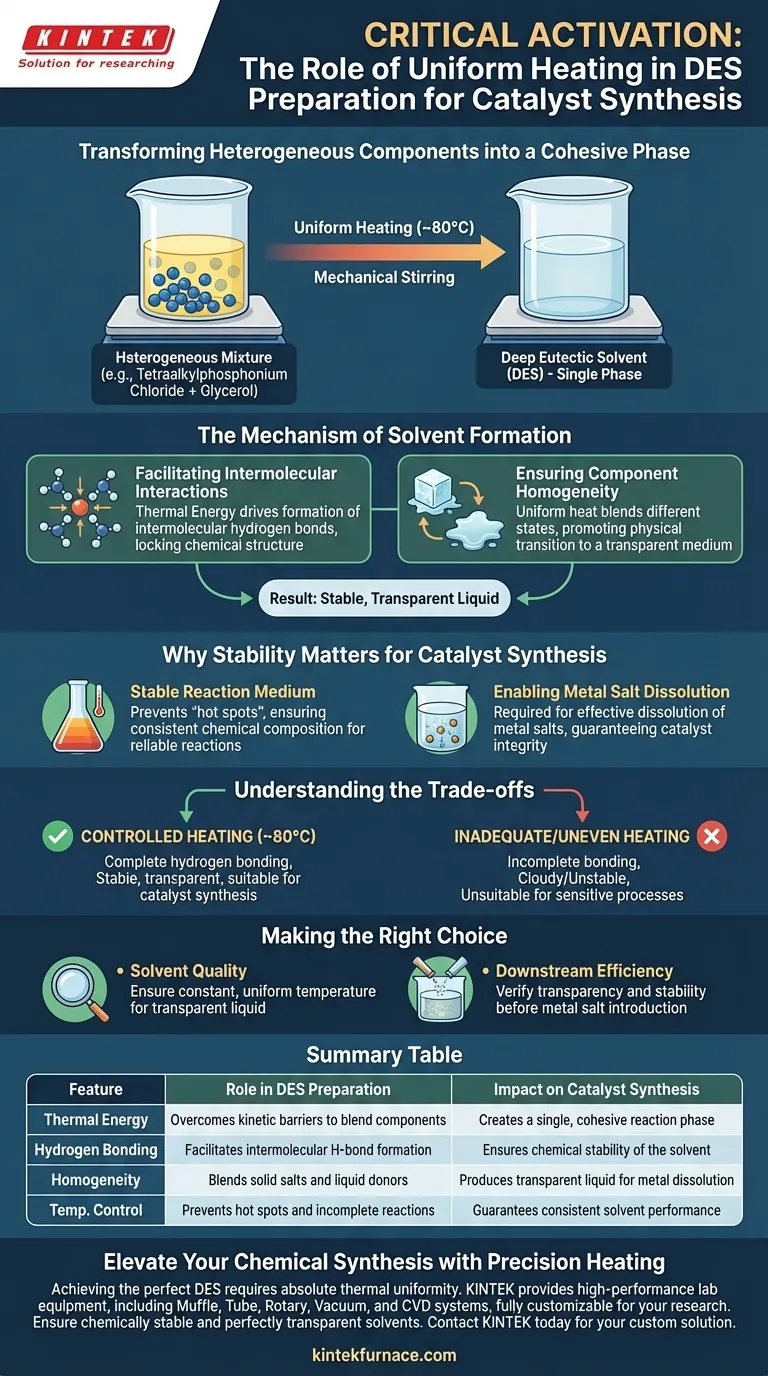
Providing a uniform heating environment is the critical activation step in the preparation of deep eutectic solvents (DES). By maintaining a controlled temperature, typically around 80°C, you ensure that distinct components—such as tetraalkylphosphonium chloride and glycerol—transition from a heterogeneous mixture into a single, cohesive phase. This thermal energy, often aided by mechanical stirring, is necessary to overcome kinetic barriers and facilitate thorough mixing.
Controlled heating provides the necessary energy to drive the formation of intermolecular hydrogen bonds between components. This results in a chemically stable, transparent liquid that is essential for the subsequent dissolution of metal salts in catalyst synthesis.

The Mechanism of Solvent Formation
Facilitating Intermolecular Interactions
The primary chemical function of the heating process is to drive the formation of intermolecular hydrogen bonds.
Simply mixing the components at room temperature is often insufficient to create the eutectic system. The application of heat provides the activation energy required for the hydrogen bond donors and acceptors to interact effectively, locking the chemical structure of the solvent into place.
Ensuring Component Homogeneity
Uniform heating ensures that components with different physical states (e.g., solid salts like tetraalkylphosphonium chloride and liquid hydrogen bond donors like glycerol) blend completely.
Without this thermal input, the mixture would likely remain separated or incompletely reacted. The heat promotes a physical transition that results in a transparent and uniform reaction medium, indicating that a true deep eutectic solvent has been formed.
Why Stability Matters for Catalyst Synthesis
Creating a Stable Reaction Medium
For the solvent to be useful in catalyst synthesis, it must be chemically stable.
A uniform heating profile during preparation prevents localized "hot spots" or "cold spots" that could lead to an inconsistent chemical composition. This stability is a prerequisite for the solvent's role as a reliable medium for downstream chemical reactions.
Enabling Metal Salt Dissolution
The ultimate goal of preparing these solvents is often to synthesize catalysts involving metal salts.
The reference material explicitly notes that a transparent, uniform solvent is required to dissolve metal salts effectively. If the heating environment is inconsistent, the solvent may fail to solubilize these salts, compromising the integrity and performance of the final catalyst.
Understanding the Trade-offs
The Necessity of Control
While heating is essential, the reference emphasizes that the environment must be controlled (e.g., maintaining a steady 80°C).
Inadequate or uneven heating leads to incomplete hydrogen bonding, resulting in a cloudy or unstable mixture. Conversely, while not explicitly detailed in the text, implies that deviating from the optimal controlled temperature could fail to produce the required transparency, rendering the solvent unsuitable for the sensitive process of catalyst synthesis.
Making the Right Choice for Your Goal
To ensure the success of your catalyst synthesis, apply the following principles during solvent preparation:
- If your primary focus is Solvent Quality: Ensure the heating source provides a constant, uniform temperature (e.g., 80°C) to guarantee the formation of a transparent, homogeneous liquid.
- If your primary focus is Downstream Efficiency: Verify that the solvent is fully transparent and stable before introducing metal salts to ensure complete dissolution.
Uniform thermal input is the foundational step that transforms raw components into a functional, stable medium for advanced chemical synthesis.
Summary Table:
| Feature | Role in DES Preparation | Impact on Catalyst Synthesis |
|---|---|---|
| Thermal Energy | Overcomes kinetic barriers to blend components | Creates a single, cohesive reaction phase |
| Hydrogen Bonding | Facilitates intermolecular H-bond formation | Ensures chemical stability of the solvent |
| Homogeneity | Blends solid salts and liquid donors | Produces transparent liquid for metal dissolution |
| Temp. Control | Prevents hot spots and incomplete reactions | Guarantees consistent solvent performance |
Elevate Your Chemical Synthesis with Precision Heating
Achieving the perfect deep eutectic solvent requires more than just heat; it requires absolute thermal uniformity. KINTEK provides high-performance lab equipment designed to meet the rigorous demands of advanced catalyst synthesis.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces, all fully customizable for your unique research needs. Ensure your solvents are chemically stable and perfectly transparent with our industry-leading technology.
Contact KINTEK today to find your custom heating solution
Visual Guide
References
- Chenyun Zhang, Jiahao Wang. Preparation of P‐Doped Ni Catalyst Using Deep Eutectic Solvents and Its Excellent Hydrogen Evolution Performance in Water Splitting. DOI: 10.1002/open.202500023
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What considerations lead to the selection of a corundum crucible for CVD sulfurization? Ensure Peak Sample Purity
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- Why is a laboratory vacuum degasser necessary for biochar? Ensure Accurate BET Structural Characterization
- What is the role of quartz capillaries in the vacuum sealing process of sulfur? Enhance Purity and In-Situ Analysis
- Why is zirconia grinding media preferred for NN-10ST ceramic powders? Ensure Purity & Dielectric Performance
- Why is the low thermal expansion of quartz important for laboratory applications? Ensure Safety and Precision in High-Heat Experiments
- What is the function of a graphite crucible in iron ore softening tests? Simulate Blast Furnace Conditions Perfectly
- What are the key mechanical properties of alumina tubes? Uncover High-Strength, Wear-Resistant Solutions



















