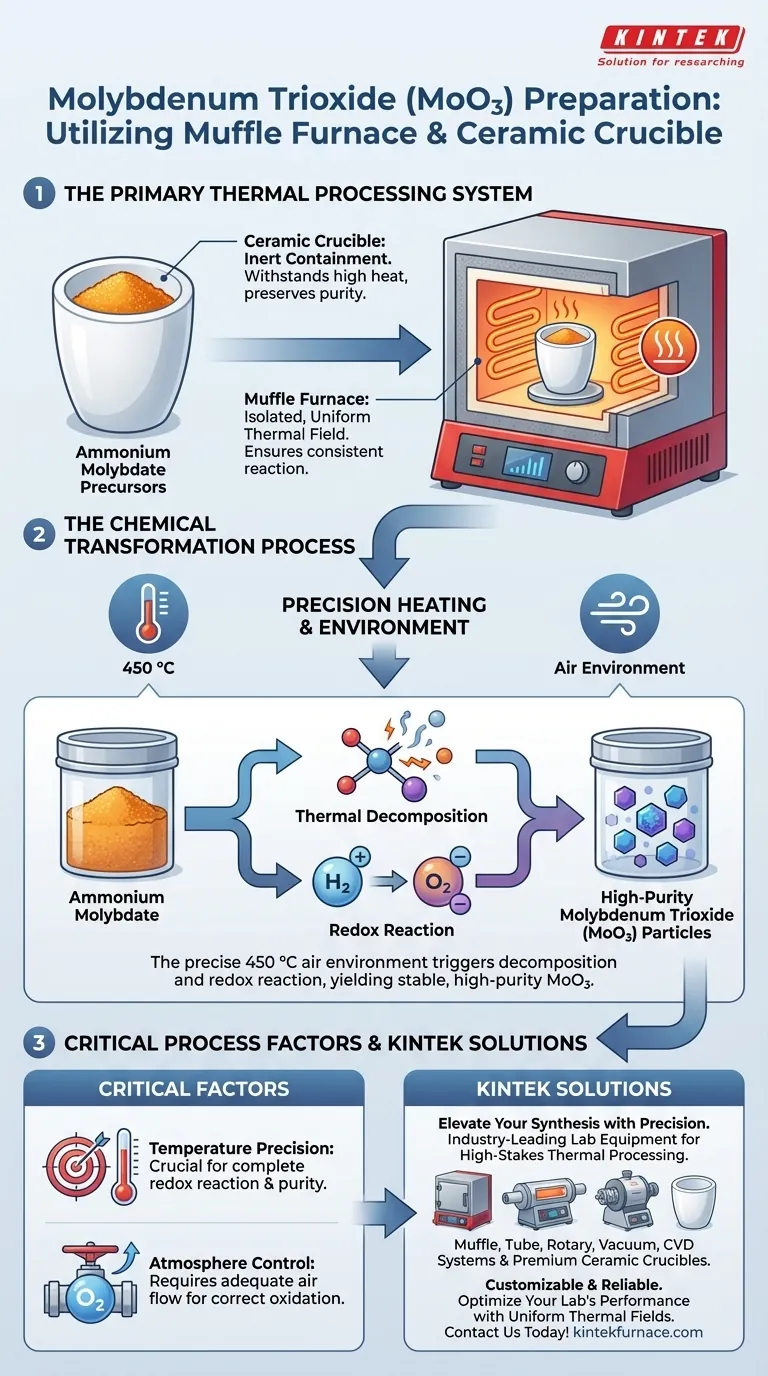
In the preparation of molybdenum trioxide (MoO3), the muffle furnace and ceramic crucible function as the primary thermal processing system required to convert raw precursors into the final oxide. Specifically, they are utilized to facilitate the thermal decomposition of ammonium molybdate precursors in an air environment at a precise temperature of 450 °C.
The combination of a ceramic crucible and a muffle furnace creates a controlled, uniform thermal field that triggers a critical redox reaction, ensuring the resulting molybdenum trioxide particles achieve the high purity necessary for heterostructure synthesis.

The Role of the Equipment
The Function of the Ceramic Crucible
The ceramic crucible serves as the inert containment vessel for the raw materials.
It holds the ammonium molybdate precursors during the heating process.
Its material properties allow it to withstand high temperatures without reacting chemically with the precursor, preserving the purity of the final product.
The Function of the Muffle Furnace
The muffle furnace provides the isolated, controlled heating environment necessary for the reaction.
It is designed to generate a uniform thermal field, ensuring that the entire sample within the crucible experiences the same temperature simultaneously.
This uniformity is essential for consistency, preventing uneven reaction rates across the sample batch.
The Chemical Transformation Process
Triggering Thermal Decomposition
The equipment is utilized to drive a specific chemical breakdown known as thermal decomposition.
By maintaining an air environment at 450 °C, the furnace supplies the energy required to break the bonds of the ammonium molybdate.
This process eliminates volatile components from the precursor, leaving behind the molybdenum oxide structure.
Facilitating the Redox Reaction
Beyond simple drying, the thermal energy triggers a distinct redox (reduction-oxidation) reaction.
This chemical shift alters the oxidation state of the material, converting the precursor into stable molybdenum trioxide (MoO3).
The precise temperature control of the furnace ensures this reaction proceeds to completion, resulting in high-purity particles.
Critical Process Factors and Trade-offs
Temperature Precision vs. Reaction Quality
The synthesis relies heavily on maintaining the specific target temperature of 450 °C.
A muffle furnace is advantageous because it isolates the sample from direct fuel combustion, but it must be well-calibrated.
If the thermal field is not uniform, the redox reaction may be incomplete, leading to impurities in the final particles that could compromise subsequent heterostructure synthesis.
Atmosphere Control
The process explicitly requires an air environment to facilitate the correct oxidation.
While some synthesis methods require inert gases (like argon), this specific protocol utilizes the ambient oxygen present in the air.
Users must ensure the furnace allows for adequate air interaction rather than sealing the chamber under a vacuum or inert gas, which would inhibit the formation of MoO3.
Making the Right Choice for Your Goal
To ensure the successful preparation of molybdenum trioxide, align your equipment usage with your specific objectives:
- If your primary focus is high purity: Ensure the ceramic crucible is clean and chemically inert to prevent contamination during the 450 °C heating cycle.
- If your primary focus is reaction consistency: Verify that your muffle furnace is calibrated to provide a strictly uniform thermal field to guarantee the redox reaction occurs evenly throughout the precursor mass.
The successful synthesis of MoO3 relies not just on the materials, but on the precise thermal environment established by the furnace and crucible.
Summary Table:
| Component | Role in MoO3 Preparation | Key Process Requirements |
|---|---|---|
| Ceramic Crucible | Inert containment of precursors | Chemical stability, high-temperature resistance |
| Muffle Furnace | Provides isolated, uniform heat | Precise 450 °C control, uniform thermal field |
| Atmosphere | Facilitates oxidation (Redox) | Consistent air environment (non-vacuum) |
| Precursor | Ammonium Molybdate | Controlled thermal decomposition |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a successful redox reaction and a contaminated sample. KINTEK provides industry-leading lab equipment tailored for high-stakes thermal processing.
Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with premium ceramic crucibles—all fully customizable for your unique research needs. Whether you are synthesizing molybdenum trioxide or developing advanced heterostructures, our technology ensures a uniform thermal field and unmatched reliability.
Ready to optimize your lab's performance? Contact us today to find the perfect solution!
Visual Guide
References
- Muhammad Ahsan Naseeb, Amir Waseem. Molybdenum carbide supported metal–organic framework-derived Ni, Co phosphosulphide heterostructures as efficient OER and HER catalysts. DOI: 10.1039/d5na00510h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the purpose of using a high-temperature muffle furnace for thermal etching of ceramic samples? Expert Insights
- Why is atmosphere control important in a Muffle furnace? Unlock Precise Material Processing
- What maintenance practices are recommended for muffle furnaces? Ensure Longevity and Precision in Your Lab
- What is the technical role of a muffle furnace in dyeing sludge ash preparation? Optimize Pozzolanic Activation
- What industries commonly use electric muffle furnaces? Essential for Precise High-Temp Processing
- What is a benchtop furnace and its common types? Choose the Right One for Your Lab
- What are the key differences between a muffle furnace and a vacuum furnace? Choose the Right Furnace for Your Lab
- How does a muffle furnace differ from a regular oven or furnace? Discover Precision Heating Solutions



















