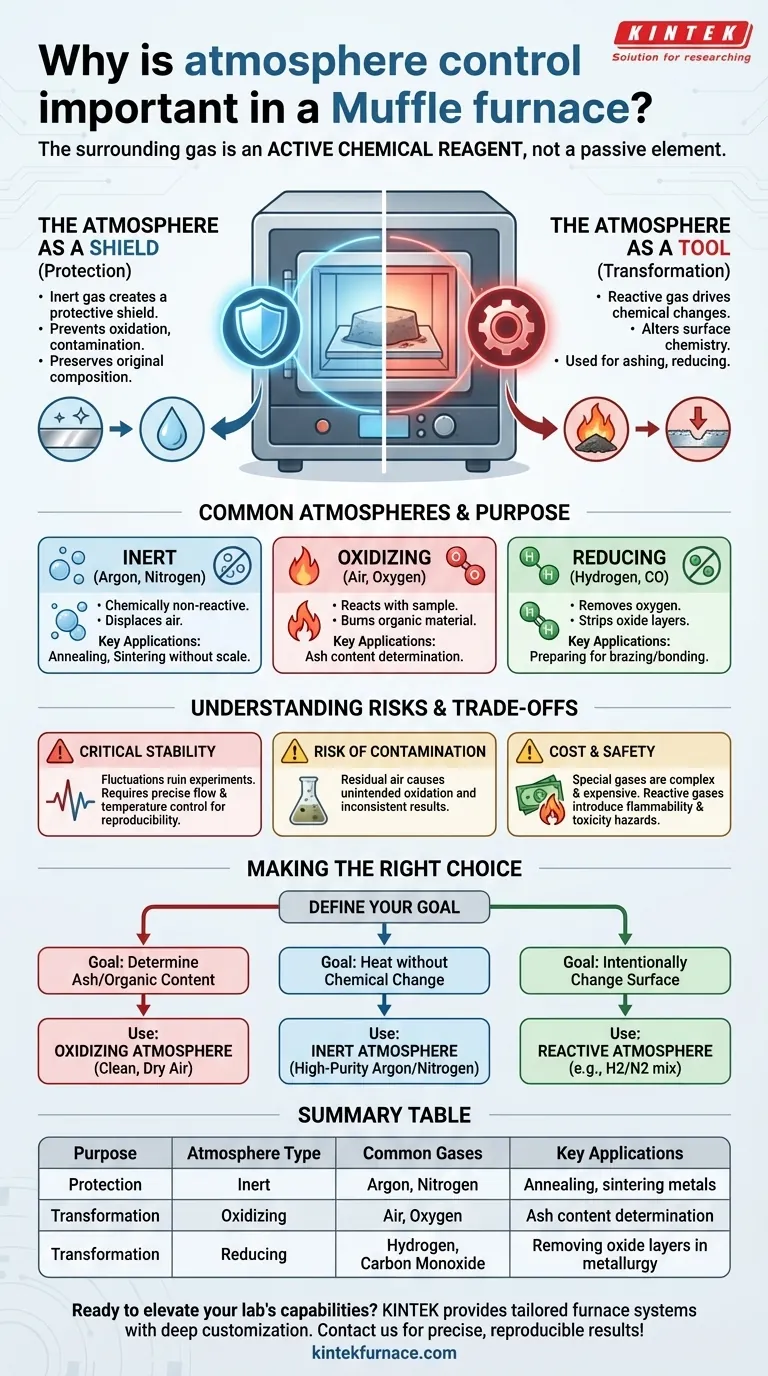
In short, atmosphere control is critical because the gas surrounding your sample inside a muffle furnace is not a passive element; it is an active chemical reagent. This controlled atmosphere dictates whether your material is protected from change, intentionally transformed, or simply burned away, directly determining the outcome of your heat treatment process.
The core purpose of atmosphere control is to manage chemical reactions at high temperatures. It allows you to either create an inert environment to prevent unwanted reactions like oxidation or to introduce a specific reactive gas to deliberately alter the material's surface and properties.

The Two Roles of a Furnace Atmosphere
A muffle furnace's design separates the sample from the heating elements, allowing the chamber's gaseous environment to be precisely managed. This atmosphere serves one of two fundamental purposes: protection or transformation.
The Atmosphere as a Shield (Protection)
Many materials are highly reactive with oxygen, especially at elevated temperatures. Introducing an inert gas creates a protective shield around the sample.
This prevents oxidation, contamination, and other unwanted surface reactions that would otherwise occur in ambient air. The goal here is to heat the material while preserving its original chemical composition.
The Atmosphere as a Tool (Transformation)
Conversely, you can use the atmosphere to intentionally drive chemical changes. By introducing a specific reactive gas, you can precisely alter the surface chemistry of a material.
This is the principle behind processes like ashing, where an oxygen-rich atmosphere is used to burn off organic compounds, or reducing, where a hydrogen-rich atmosphere is used to remove oxygen from metal oxides.
Common Atmospheres and Their Purpose
The choice of gas is entirely dependent on your desired outcome. Each one provides a unique chemical environment.
Inert Atmospheres (Argon, Nitrogen)
These gases are chemically non-reactive. They are used to displace air and prevent the sample from reacting with oxygen or moisture during heating. This is common for processes like annealing or sintering metals without forming a scale or oxide layer.
Oxidizing Atmospheres (Air, Oxygen)
This is the most common and simple atmosphere. It is used when the goal is to react the sample with oxygen. The primary application is ash content determination, where all organic material must be completely burned away, leaving only inorganic ash.
Reducing Atmospheres (Hydrogen, Carbon Monoxide)
These atmospheres are used to remove oxygen from a material. In metallurgy, a reducing atmosphere can strip oxide layers from a metal's surface, a critical step in preparing materials for brazing or other bonding processes.
Understanding the Trade-offs and Risks
While powerful, atmosphere control introduces complexity and requires careful management. Missteps can easily compromise your results.
The Critical Need for Stability
Sudden fluctuations in either atmosphere composition or temperature can ruin an experiment. A stable, controlled atmosphere requires precise flow rates, and this must be paired with stable temperature control—including ramp rates, hold times, and cooling periods—to ensure results are accurate and reproducible.
Risk of Contamination
If the furnace chamber is not properly purged, residual air (specifically oxygen and moisture) can remain and cause unintended, low-level oxidation. This can subtly alter material properties and lead to inconsistent results.
Cost and Safety
Using specialized gases like purified argon or hydrogen is more complex and expensive than using ambient air. It requires additional equipment like gas cylinders, regulators, and flow controllers. Furthermore, reactive gases like hydrogen and carbon monoxide introduce significant safety hazards (flammability and toxicity) that must be properly managed.
Making the Right Choice for Your Goal
Selecting the correct atmosphere begins with defining the goal of your heat-treatment process.
- If your primary focus is determining ash or organic content: Use a simple oxidizing atmosphere of clean, dry air to ensure complete combustion.
- If your primary focus is heating a material without changing its chemistry: Use a protective, inert atmosphere like high-purity argon or nitrogen to prevent oxidation.
- If your primary focus is intentionally changing a material's surface: Use a specific reactive atmosphere, such as a hydrogen/nitrogen mix for reduction or a carbon-rich gas for carburization.
Ultimately, mastering atmosphere control transforms the muffle furnace from a simple oven into a precise tool for materials engineering.
Summary Table:
| Purpose | Atmosphere Type | Common Gases | Key Applications |
|---|---|---|---|
| Protection | Inert | Argon, Nitrogen | Annealing, sintering metals |
| Transformation | Oxidizing | Air, Oxygen | Ash content determination |
| Transformation | Reducing | Hydrogen, Carbon Monoxide | Removing oxide layers in metallurgy |
Ready to elevate your lab's capabilities with advanced high-temperature solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide diverse laboratories with tailored furnace systems. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization to meet your unique experimental needs. Don't let atmosphere control challenges hold you back—contact us today to discuss how we can help you achieve precise, reproducible results!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















