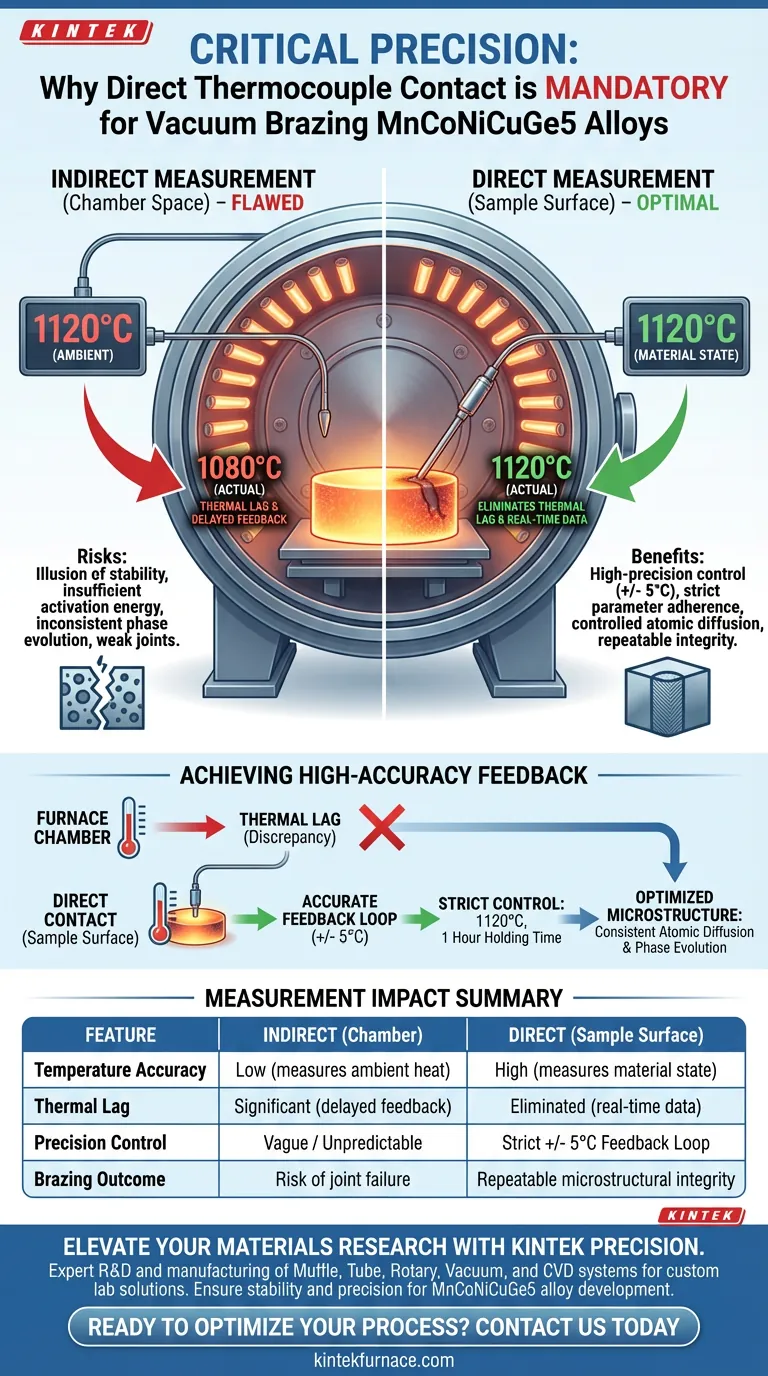
Direct contact between the thermocouple and the sample surface is mandatory because it eliminates the significant thermal lag that exists between the furnace chamber space and the MnCoNiCuGe5 alloy itself. By measuring the sample directly, you ensure the system acts upon the actual temperature of the material rather than the ambient environment, enabling a high-precision feedback loop with an accuracy of +/- 5 degrees Celsius.
Core Takeaway To guarantee the repeatability of atomic diffusion and phase evolution, you cannot rely on ambient furnace temperature. You must anchor your thermal control to the sample's physical surface to achieve the strict precision required for successful vacuum brazing.
The Physics of Thermal Precision
Eliminating Temperature Lag
In vacuum brazing, there is often a discrepancy between the temperature of the heating elements (the furnace chamber) and the actual temperature of the sample.
If you measure the chamber space, you are measuring the potential for heat, not the heat absorbed by the alloy. Fixing the thermocouple directly to the sample bridges this gap, removing the temperature lag from the data equation.
Achieving High-Accuracy Feedback
Direct surface contact converts a general heating process into a precision operation.
This specific configuration allows for a control accuracy of +/- 5 degrees Celsius. Without this tight feedback loop, the actual temperature of the alloy could drift outside the optimal processing window, even if the furnace controller displays the correct setpoint.
Why Control Matters for MnCoNiCuGe5 Alloys
Strictly Controlling Brazing Parameters
High-entropy alloys like MnCoNiCuGe5 require exacting conditions to process correctly.
The primary reference highlights a specific brazing temperature of 1120 degrees Celsius and a holding time of 1 hour. Direct thermocouple attachment ensures these parameters are met by the material itself, not just the surrounding air.
Ensuring Process Repeatability
The ultimate goal of this precision is to control the microstructure of the joint.
Strict adherence to the temperature and time profiles ensures the repeatability of atomic diffusion across the joint interface. Furthermore, it regulates phase evolution, ensuring that the resulting material properties are consistent from one experiment to the next.
Understanding the Risks of Indirect Measurement
The Illusion of Stability
A common pitfall in high-temperature experiments is assuming the furnace temperature equals the sample temperature.
If you rely on the chamber thermocouple, you may believe the sample has reached 1120 degrees Celsius when it is actually significantly cooler. This results in insufficient activation energy for the necessary diffusion processes.
Compromising Joint Integrity
The trade-off for easier setup (not fixing the thermocouple to the sample) is a complete loss of experimental validity.
If the temperature fluctuates beyond the +/- 5 degree tolerance due to lag, the phase evolution within the brazed joint becomes unpredictable. This leads to weak joints and data that cannot be replicated in future studies.
Ensuring Success in Vacuum Brazing
To replicate the success of atomic diffusion and phase formation in MnCoNiCuGe5 alloys, you must prioritize the source of your thermal data.
- If your primary focus is Experimental Accuracy: Fix the thermocouple to the sample to guarantee the reading reflects the material's actual state within +/- 5 degrees Celsius.
- If your primary focus is Microstructural Control: Use direct thermal feedback to strictly enforce the 1120 degrees Celsius setpoint and 1-hour holding time required for consistent phase evolution.
Precision in measurement is the only path to predictability in material performance.
Summary Table:
| Feature | Indirect Measurement (Chamber) | Direct Measurement (Sample Surface) |
|---|---|---|
| Temperature Accuracy | Low (measures ambient heat) | High (measures material state) |
| Thermal Lag | Significant (delayed feedback) | Eliminated (real-time data) |
| Precision Control | Vague / Unpredictable | Strict +/- 5°C Feedback Loop |
| Process Impact | Inconsistent phase evolution | Controlled atomic diffusion |
| Brazing Outcome | Risk of joint failure | Repeatable microstructural integrity |
Elevate Your Materials Research with KINTEK Precision
Achieve the strict thermal control required for high-entropy alloy development with KINTEK’s advanced heating solutions. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique experimental needs. Whether you are brazing MnCoNiCuGe5 alloys or developing new materials, our high-temperature furnaces provide the stability and precision you demand.
Ready to optimize your vacuum brazing process? Contact us today to find your custom lab solution.
Visual Guide
References
- S.V. Maksymova, V.V. Voronov. Structure formation of seams using high-entropic brazing filler metal MnCoNiCuGe5. DOI: 10.21203/rs.3.rs-7260180/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- How are vacuum furnaces applied in the semiconductor industry? Essential for High-Purity Chip Manufacturing
- Why is a vacuum drying oven set to 70 °C for g-C3N4/Bi2WO6? Optimize Your Photocatalyst Post-Processing
- What is the function of a high-pressure stainless steel autoclave in hydrothermal carbonization? Unlock Superior Carbon
- What are the common materials used for constructing the hot zone in vacuum furnaces? Choose the Best for Your High-Temp Needs
- What advantages does vacuum brazing offer over other methods? Achieve Superior Joint Quality and Efficiency
- How does vacuum or protective atmosphere melting improve the quality of aerospace materials? Achieve Superior Purity and Performance
- What is the typical working vacuum degree for most heat treatment vacuum furnaces? Optimize Your Process with the Right Vacuum Level
- How are temperature and pressure controlled in vacuum sintering? Achieve Precise Material Densification and Performance



















