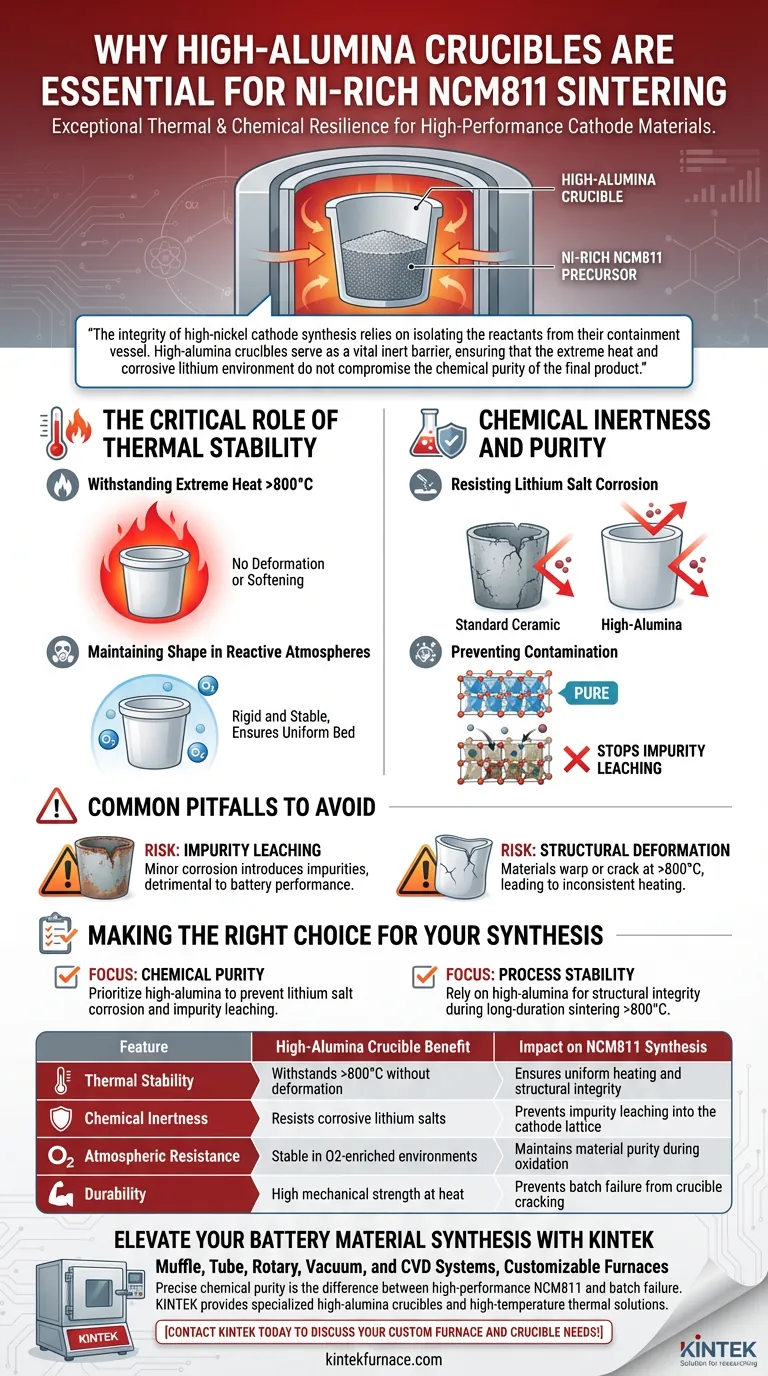
High-alumina crucibles are essential for the solid-state sintering of Ni-Rich NCM811 due to their exceptional thermal and chemical resilience. They withstand temperatures exceeding 800°C without deformation and, more importantly, resist corrosion from reactive lithium salts, preventing the leaching of impurities that would otherwise degrade the cathode material.
The integrity of high-nickel cathode synthesis relies on isolating the reactants from their containment vessel. High-alumina crucibles serve as a vital inert barrier, ensuring that the extreme heat and corrosive lithium environment do not compromise the chemical purity of the final product.

The Critical Role of Thermal Stability
Withstanding Extreme Heat
The synthesis of NCM811 requires prolonged exposure to temperatures exceeding 800°C. High-alumina crucibles possess the thermal resistance necessary to endure this heat treatment without softening or losing structural integrity.
Maintaining Shape in Reactive Atmospheres
Sintering typically occurs in air or oxygen-enriched atmospheres to facilitate the correct oxidation state of the metals. High-alumina ceramics remain rigid and stable in these environments, ensuring the sample bed remains uniform throughout the process.
Chemical Inertness and Purity
Resisting Lithium Salt Corrosion
A specific challenge in synthesizing cathode materials is the presence of lithium salts, which become highly corrosive at sintering temperatures. Standard ceramic materials may react with these salts, leading to the rapid degradation of the crucible walls.
Preventing Contamination
High-alumina material is chemically stable enough to resist this corrosive attack. By preventing the crucible from breaking down, it stops foreign elements from leaching into the cathode material, guaranteeing the chemical purity of the NCM811 samples.
Common Pitfalls to Avoid
The Risk of Impurity Leaching
Using crucibles with lower chemical resistance is a frequent cause of batch failure. Even minor corrosion can introduce impurities into the crystal lattice of the cathode, which can severely detrimental to the battery's electrochemical performance.
Structural Deformation
Materials that cannot withstand the >800°C requirement may warp or crack during the long-duration heat treatment. This deformation can lead to inconsistent heating profiles or physical loss of the valuable precursor material.
Making the Right Choice for Your Synthesis
To maximize the quality of your NCM811 cathode material, select your crucible based on your specific processing parameters:
- If your primary focus is Chemical Purity: Prioritize high-alumina content to strictly prevent lithium salt corrosion and subsequent impurity leaching.
- If your primary focus is Process Stability: Rely on high-alumina vessels to maintain structural integrity during long-duration sintering above 800°C.
By selecting the correct vessel, you ensure that the limiting factor of your battery performance is the chemistry itself, not the equipment used to create it.
Summary Table:
| Feature | High-Alumina Crucible Benefit | Impact on NCM811 Synthesis |
|---|---|---|
| Thermal Stability | Withstands >800°C without deformation | Ensures uniform heating and structural integrity |
| Chemical Inertness | Resists corrosive lithium salts | Prevents impurity leaching into the cathode lattice |
| Atmospheric Resistance | Stable in O2-enriched environments | Maintains material purity during oxidation |
| Durability | High mechanical strength at heat | Prevents batch failure from crucible cracking |
Elevate Your Battery Material Synthesis with KINTEK
Precise chemical purity is the difference between high-performance NCM811 and batch failure. KINTEK provides the specialized high-alumina crucibles and high-temperature thermal solutions needed to master the challenges of lithium-salt corrosion and extreme heat.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable laboratory furnaces designed for your unique synthesis requirements. Don't let container contamination compromise your electrochemical performance.
Contact KINTEK today to discuss your custom furnace and crucible needs!
Visual Guide
References
- Liang‐Yin Kuo, Payam Kaghazchi. Doping‐Induced Surface and Grain Boundary Effects in Ni‐Rich Layered Cathode Materials. DOI: 10.1002/smll.202307678
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is the pore size of refractory materials significant? Unlocking Precision in Bubble Formation and Oxygen Impact
- What information does laboratory XRD provide for Gallium Sulfide? Master GaS Single Crystal Characterization
- Why are stainless steel tubes used during the cooling and heat treatment stages of Ti–Nb–Si alloys? Key Cooling Insights
- What are the primary functions of high-purity graphite crucibles? Optimize Mg-Zn-xSr Alloy Purity and Efficiency
- What are the technical considerations for selecting a graphite crucible? Expert Insights for Molten Salt Electrolysis
- What role does a high-precision lab stirring device play in KR experiments? Optimizing Desulfurization Simulation
- What precautions should be taken when using the alumina furnace tube for the first time? Ensure Safe Initial Use with Proper Conditioning
- How does a planetary ball mill prepare precursors for furnaces? Unlock Nano-Scale Precision for High-Temp Success



















