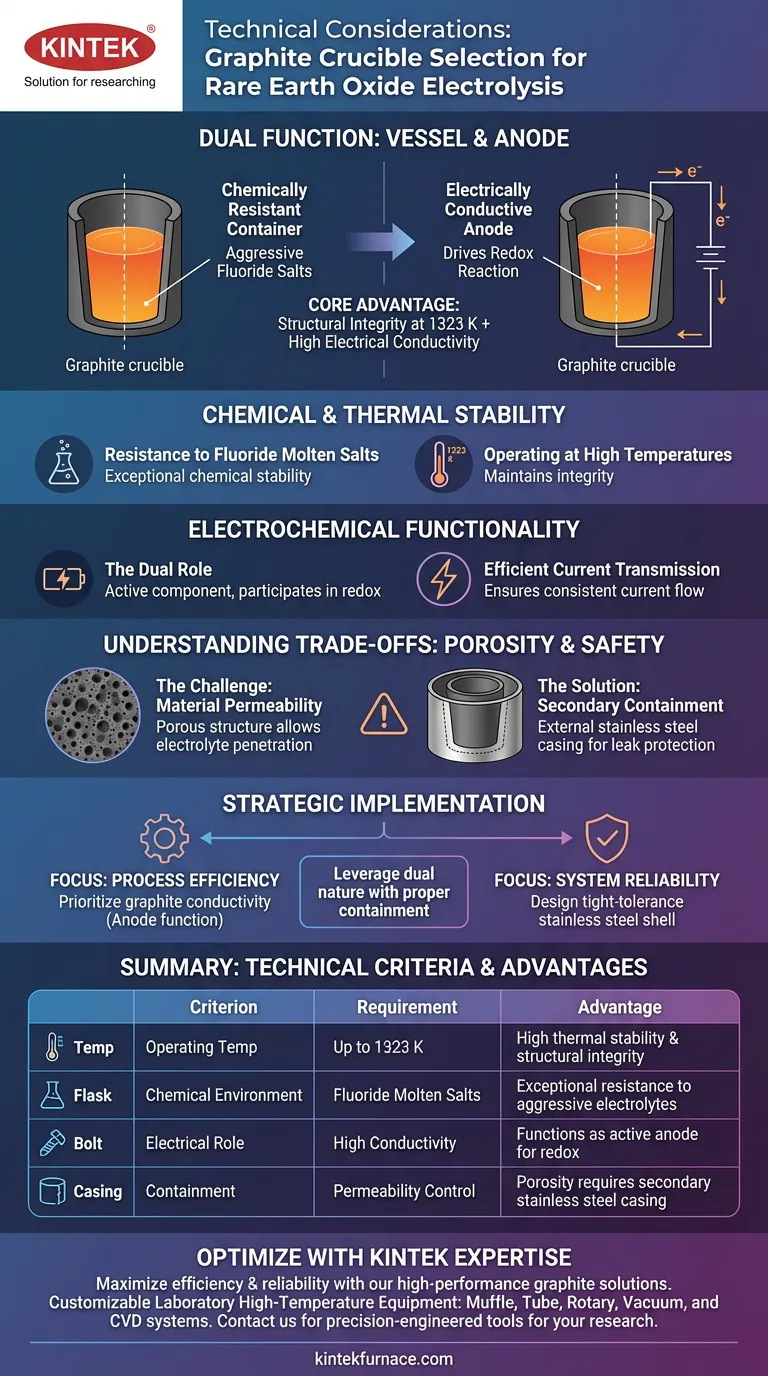
Selecting a graphite crucible for rare earth oxide electrolysis is primarily a decision driven by the material's ability to perform a dual function in aggressive environments. It serves simultaneously as a chemically resistant container for high-temperature fluoride salts and as the electrically conductive anode required to drive the redox reaction.
The core technical advantage of graphite in this application is its capacity to combine structural integrity at 1323 K with high electrical conductivity. However, its effective implementation requires a secondary stainless steel barrier to mitigate risks associated with the material's inherent porosity.

Chemical and Thermal Stability
Resistance to Fluoride Molten Salts
The primary challenge in rare earth electrolysis is containing the highly corrosive electrolyte. Graphite acts as the reaction vessel because it exhibits exceptional chemical stability when in contact with fluoride molten salt systems.
Operating at High Temperatures
The electrolysis process demands extreme thermal conditions to maintain the salt in a liquid state. Graphite maintains its structural integrity and chemical properties even when subjected to operating temperatures of 1323 K.
Electrochemical Functionality
The Dual Role of the Crucible
Unlike standard inert vessels, a graphite crucible is an active component of the electrolytic cell. It functions as the anode, directly participating in the redox reaction necessary to separate the rare earth elements.
Efficient Current Transmission
Successful electrolysis relies on stable energy delivery throughout the system. Graphite’s high electrical conductivity ensures consistent current transmission through the molten salt, facilitating an efficient reaction.
Understanding the Trade-offs: Porosity and Safety
The Challenge of Material Permeability
While graphite is chemically stable, it is not perfectly impermeable. The porous structure of graphite presents a technical risk, as the molten electrolyte can penetrate the vessel walls over time.
The Requirement for Secondary Containment
To counteract the risk of penetration, the graphite crucible cannot stand alone. Reliability is reinforced by encasing the graphite in an external stainless steel container, which provides necessary secondary protection against leaks.
Strategic Implementation for Electrolysis Design
To ensure a safe and efficient electrolysis process, you must balance the material's electrochemical benefits with its physical limitations.
- If your primary focus is Process Efficiency: Prioritize the graphite crucible for its conductivity, utilizing its ability to act as the anode to simplify the internal cell design.
- If your primary focus is System Reliability: Design the external stainless steel shell with tight tolerances to account for graphite's porosity and potential electrolyte penetration.
Leveraging graphite’s dual nature offers a streamlined technical solution, provided the containment architecture accounts for its physical permeability.
Summary Table:
| Technical Criterion | Key Requirement | Advantage of Graphite |
|---|---|---|
| Operating Temp | Up to 1323 K | High thermal stability and structural integrity |
| Chemical Environment | Fluoride Molten Salts | Exceptional resistance to aggressive electrolytes |
| Electrical Role | High Conductivity | Functions as the active anode for redox reactions |
| Containment | Permeability Control | Porosity requires secondary stainless steel casing |
Optimize Your Rare Earth Electrolysis with KINTEK Expertise
Maximize process efficiency and system reliability with our high-performance graphite solutions. Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory high-temperature equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique molten salt electrolysis needs.
Whether you require specialized graphite crucibles or advanced thermal containment systems, our team is ready to deliver precision-engineered tools for your research. Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Greenhouse Gas Emissions from Molten Fluoride Electrolysis Composed of Raw and Magnet Recycling Derived Oxides: A Comparative Study. DOI: 10.3390/ma18010184
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why are alumina crucibles used for CoNb2O6 synthesis? Ensure High-Purity Ceramic Powder Production
- What role do contact thermocouples play during the high-temperature annealing experiments of oriented silicon steel?
- What is the function of a laboratory hydraulic press in Al-Cr-Cu-Fe-Mn-Ni alloy formation? Maximize Green Strength
- What is the primary function of a high-alumina powder crucible? Ensure Purity in Maraging Steel Pre-treatment
- How does a laboratory drying oven ensure the structural stability of microcapsule granules? Expert Drying Guide
- How does surface finish impact the performance of alumina ceramic furnace tubes? Boost Purity and Efficiency
- What is the core function of a high-purity quartz crucible? Ensuring Success in Czochralski Silicon Growth
- What are the primary functions of multilayer fixtures within a lithium battery vacuum oven? Optimize Your Drying Process



















