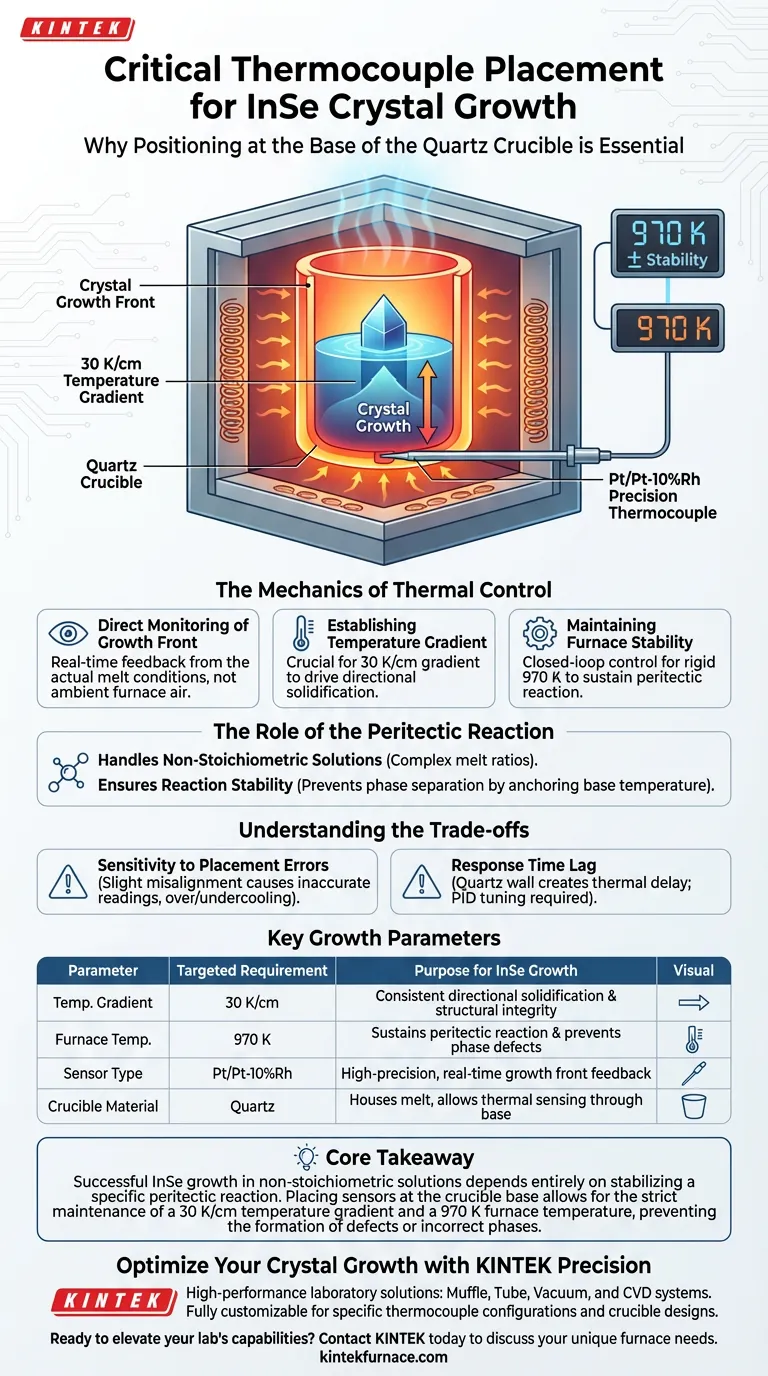
The placement of precision thermocouples at the base of the quartz crucible is critical because it provides direct, real-time feedback on the temperature conditions at the actual crystal growth front. This specific positioning is the only way to accurately regulate the thermal environment needed to sustain the delicate peritectic reaction required for Indium Selenide (InSe) crystallization.
Core Takeaway Successful InSe growth in non-stoichiometric solutions depends entirely on stabilizing a specific peritectic reaction. Placing sensors at the crucible base allows for the strict maintenance of a 30 K/cm temperature gradient and a 970 K furnace temperature, preventing the formation of defects or incorrect phases.

The Mechanics of Thermal Control
Direct Monitoring of the Growth Front
To grow high-quality crystals, you must monitor the exact point where solidification occurs. Placing Pt/Pt-10%Rh precision thermocouples at the base of the crucible places the sensor as close to the crystal growth front as possible.
This allows for the collection of data that reflects the actual conditions of the melt, rather than the ambient temperature of the furnace.
Establishing the Temperature Gradient
A precise temperature gradient is the driving force behind controlled crystallization. The data collected from the crucible base is necessary to establish a gradient of approximately 30 K/cm.
Without this specific gradient, the directional solidification of the crystal cannot be controlled effectively.
Maintaining Furnace Stability
The feedback from these thermocouples controls the power output to the furnace heaters. This closed-loop system is required to maintain a stable overall furnace temperature of approximately 970 K.
deviations from this temperature can disrupt the thermodynamic equilibrium required for growth.
The Role of the Peritectic Reaction
Handling Non-Stoichiometric Solutions
InSe crystals are grown from non-stoichiometric solutions, meaning the ratio of elements in the melt is not a simple 1:1 match with the final crystal. This requires a specific phase transformation known as a peritectic reaction.
This reaction is highly sensitive to temperature fluctuations and compositional changes in the melt.
Ensuring Reaction Stability
If the temperature at the growth front wavers, the peritectic reaction becomes unstable. This instability can lead to the inclusion of secondary phases or the cessation of crystal growth entirely.
By anchoring the control loop to the temperature at the crucible base, you ensure the reaction proceeds at a steady, predictable rate.
Understanding the Trade-offs
Sensitivity to Placement Errors
While placing thermocouples at the base provides the best data, it also introduces high sensitivity to positioning errors. A slight misalignment of the sensor can lead to a reading that does not accurately represent the thermal gradient.
This discrepancy can cause the control system to overcompensate, potentially overheating or undercooling the melt.
Response Time Lag
Even with direct contact at the base, there is a physical barrier between the sensor and the melt (the quartz crucible wall). This creates a slight thermal lag between a change in the melt temperature and the sensor reading.
Operators must tune their PID controllers to account for this lag to prevent oscillation around the target temperature of 970 K.
Making the Right Choice for Your Goal
To maximize the yield and quality of your InSe crystals, you must prioritize sensor placement based on your specific thermal requirements.
- If your primary focus is Phase Purity: Ensure the furnace temperature remains rigidly at 970 K to support the peritectic reaction without secondary phase formation.
- If your primary focus is Structural Integrity: Prioritize the 30 K/cm gradient to drive consistent directional growth and reduce internal stress.
Precision in sensor placement is not just a procedural detail; it is the foundational variable that makes the synthesis of complex InSe crystals possible.
Summary Table:
| Parameter | Targeted Requirement | Purpose for InSe Growth |
|---|---|---|
| Temperature Gradient | 30 K/cm | Drives consistent directional solidification and structural integrity. |
| Furnace Temperature | 970 K | Sustains the delicate peritectic reaction and prevents phase defects. |
| Sensor Type | Pt/Pt-10%Rh | Provides high-precision, real-time feedback from the growth front. |
| Crucible Material | Quartz | Houses the melt while allowing thermal sensing through the base. |
Optimize Your Crystal Growth with KINTEK Precision
Achieving the perfect 30 K/cm gradient for InSe crystallization requires world-class thermal stability. KINTEK provides high-performance laboratory solutions—including Muffle, Tube, Vacuum, and CVD systems—specifically engineered to meet the rigorous demands of advanced material research.
Backed by expert R&D and manufacturing, our systems are fully customizable to accommodate your specific thermocouple configurations and crucible designs, ensuring you maintain the rigid thermal control necessary for phase purity.
Ready to elevate your lab's capabilities? Contact KINTEK today to discuss your unique furnace needs with our technical specialists.
Visual Guide
References
- Min Jin, Xuechao LIU. Growth and Characterization of Large-size InSe Crystal from Non-stoichiometric Solution <i>via</i> a Zone Melting Method. DOI: 10.15541/jim20230524
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
People Also Ask
- How are silicon carbide heating elements used in chemical processing? Enhance High-Temp Corrosion Resistance
- What is thermal shock resistance and why is it important for high-temperature materials? Ensure Durability in Extreme Heat
- What role do platinum or high-temperature alloy wires play in sample suspension? Achieve Precision at 1500°C+
- How can the power of a heating element be increased? Boost Heat Output Safely with Key Methods
- What safety advantages do ceramic heating elements offer? Ensure Inherent Electrical and Thermal Protection
- What is the significance of high power density in silicon carbide heating elements? Boost Efficiency and Throughput
- Why do MoSi2 heating elements heat up quickly? Discover Their Rapid, Efficient High-Temp Performance
- How does a programmable temperature controller impact zinc recovery? Maximize Yield with B-type Thermocouple Precision













