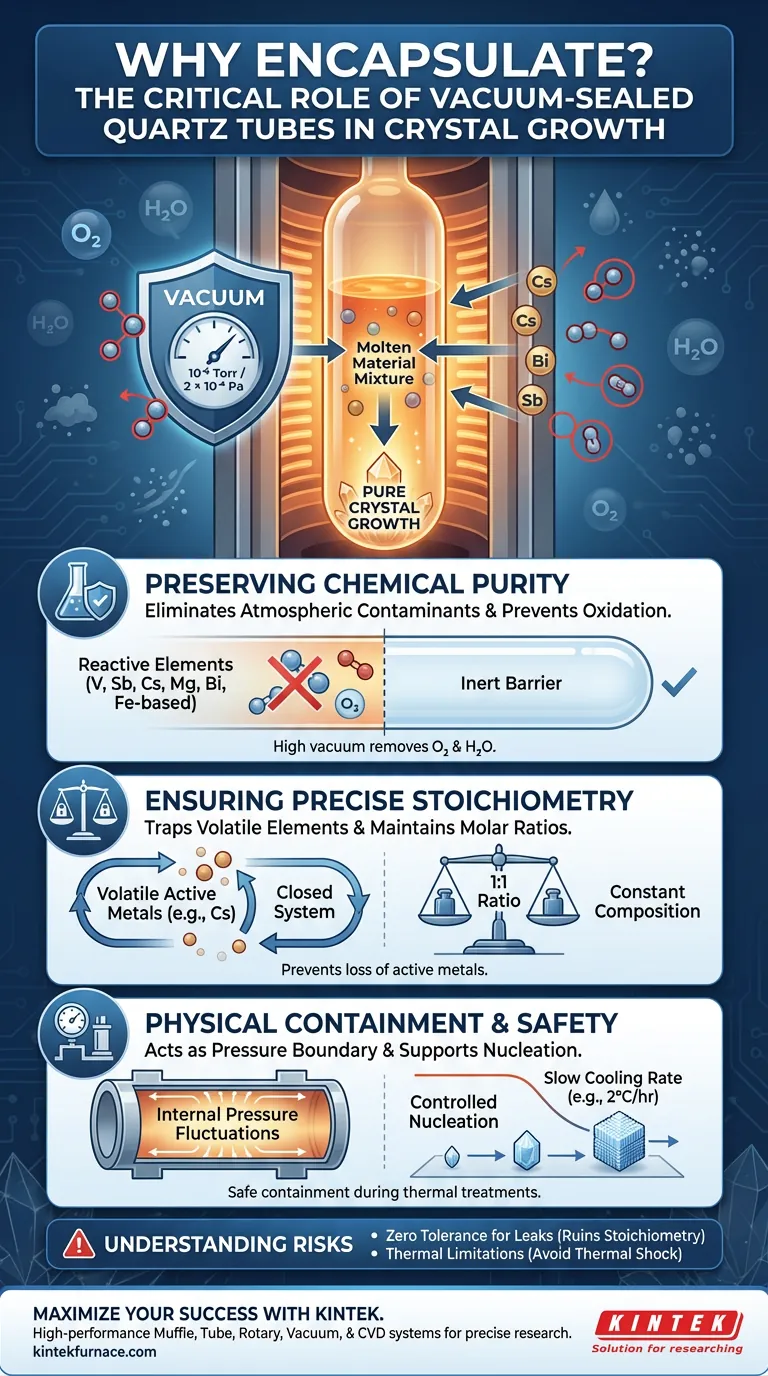
Encapsulating raw materials in a vacuum-sealed quartz tube is the fundamental defense against chemical contamination and material loss during high-temperature crystal growth. This process creates a controlled, isolated environment that prevents atmospheric gases from reacting with sensitive elements, while simultaneously stopping volatile components from evaporating. By maintaining a high vacuum (approximately $2 \times 10^{-4}$ Pa), researchers ensure the final crystal retains its precise chemical composition and structural purity.
The vacuum-sealed quartz tube acts as both a chemical shield and a containment vessel, ensuring that high-temperature synthesis occurs without oxidation or the loss of active metals, thereby preserving the material's exact stoichiometry.

Preserving Chemical Purity
High-temperature environments act as catalysts for unwanted chemical reactions. The primary function of the quartz tube is to eliminate these variables.
Eliminating Atmospheric Contaminants
At growth temperatures, many raw materials become highly reactive. Elements such as vanadium, antimony, cesium, magnesium, and bismuth will instantly oxidize if exposed to air.
The Role of High Vacuum
Creating a vacuum level of approximately $10^{-6}$ Torr (or $2 \times 10^{-4}$ Pa) removes oxygen and water vapor from the tube. This prevents the degradation of iron-based materials (like $Fe_3GeTe_2$), which are notoriously susceptible to oxidation.
Creating an Inert Boundary
The quartz tube itself provides a chemically inert barrier. It effectively isolates the melt from the outside world, ensuring that the only interactions occurring are between the intended raw materials.
Ensuring Precise Stoichiometry
Crystal growth is a game of ratios. If the proportion of elements shifts during the heating process, the desired crystal structure will not form.
Trapping Volatile Elements
Active metals, particularly cesium (Cs), have high vapor pressures and evaporate easily when heated. Without encapsulation, these elements would escape the melt and be lost to the surrounding atmosphere.
Maintaining the Molar Ratio
The sealed environment creates a closed system where no mass can enter or leave. This guarantees that the initial molar ratio (e.g., a 1:1 ratio for Indium and Bismuth) remains constant throughout the process.
Physical Containment and Safety
Beyond chemical concerns, the physical properties of the quartz tube are essential for the mechanics of the growth process, particularly in techniques like the Modified Bridgman method.
Acting as a Pressure Boundary
During long-duration thermal treatments, internal pressures can fluctuate. The sealed quartz tube acts as a pressure boundary, containing the melt safely even as temperatures rise.
Supporting Nucleation
The tube provides the necessary physical support for the melt. It withstands sustained thermal treatments (such as 220°C with slow cooling rates) and dictates the physical shape needed for controlled nucleation and growth.
Understanding the Risks
While encapsulation is necessary, it introduces specific challenges that must be managed to ensure success.
Zero Tolerance for Leaks
The system relies entirely on the integrity of the vacuum seal. Even a microscopic leak that admits a small amount of oxygen can ruin the stoichiometry of sensitive materials like $Fe_3GeTe_2$.
Thermal Limitations
While quartz is robust, it serves as a rigid physical boundary. The heating and cooling rates (e.g., $2^\circ$C per hour) must be carefully controlled to prevent thermal shock, which could shatter the tube and expose the dangerous melt to air.
Making the Right Choice for Your Goal
When designing a crystal growth experiment, understanding the specific role of the quartz encapsulation helps you prioritize your setup parameters.
- If your primary focus is Purity: Ensure your vacuum system can reliably achieve and hold $10^{-6}$ Torr to completely eliminate oxygen and water vapor.
- If your primary focus is Stoichiometry: Prioritize the seal integrity and tube volume to prevent the evaporation and segregation of volatile active metals like cesium.
Ultimately, the vacuum-sealed quartz tube is not just a container; it is an active component in defining the chemical and physical boundaries of the crystal's existence.
Summary Table:
| Feature | Purpose in Crystal Growth | Benefit to Researcher |
|---|---|---|
| High Vacuum Seal | Removes oxygen and water vapor ($2 \times 10^{-4}$ Pa) | Prevents oxidation of reactive elements |
| Chemical Isolation | Provides an inert barrier between melt and air | Ensures high chemical purity |
| Closed System | Traps volatile active metals (e.g., Cs, Bi, Sb) | Maintains precise molar ratios |
| Pressure Boundary | Contains internal pressure fluctuations | Ensures physical safety and controlled nucleation |
Maximize Your Crystal Growth Success with KINTEK
Precise stoichiometry and extreme purity are non-negotiable in materials science. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces—all fully customizable to meet your unique research needs.
Whether you are sealing quartz tubes or performing long-duration thermal treatments, our equipment provides the stability and control required for superior results. Contact us today to find your perfect heating solution!
Visual Guide
References
- Kazumi Fukushima, Shingo Yonezawa. Violation of emergent rotational symmetry in the hexagonal Kagome superconductor CsV3Sb5. DOI: 10.1038/s41467-024-47043-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What advantages do drop tube furnaces offer? Achieve Precise Control and High Efficiency
- Why Use Argon in Tube Furnaces for Titania Nanotubes? Optimize Charge Transport & Hydrogen Production
- Why are sealed quartz tubes required for TMD synthesis? Ensure Pure MoS2 & WS2 Growth
- What are the main applications of a drop tube furnace? Unlock Insights in Energy and Materials Research
- What are the key features of high temperature tube furnaces? Unlock Precision for Material Science
- What is the function of a cold tube furnace for magnesium extraction? Achieve Ultra-Pure Metal with Vacuum Evaporation
- How did the tube furnace originate and where is it commonly used today? Discover Its Evolution and Modern Applications



















