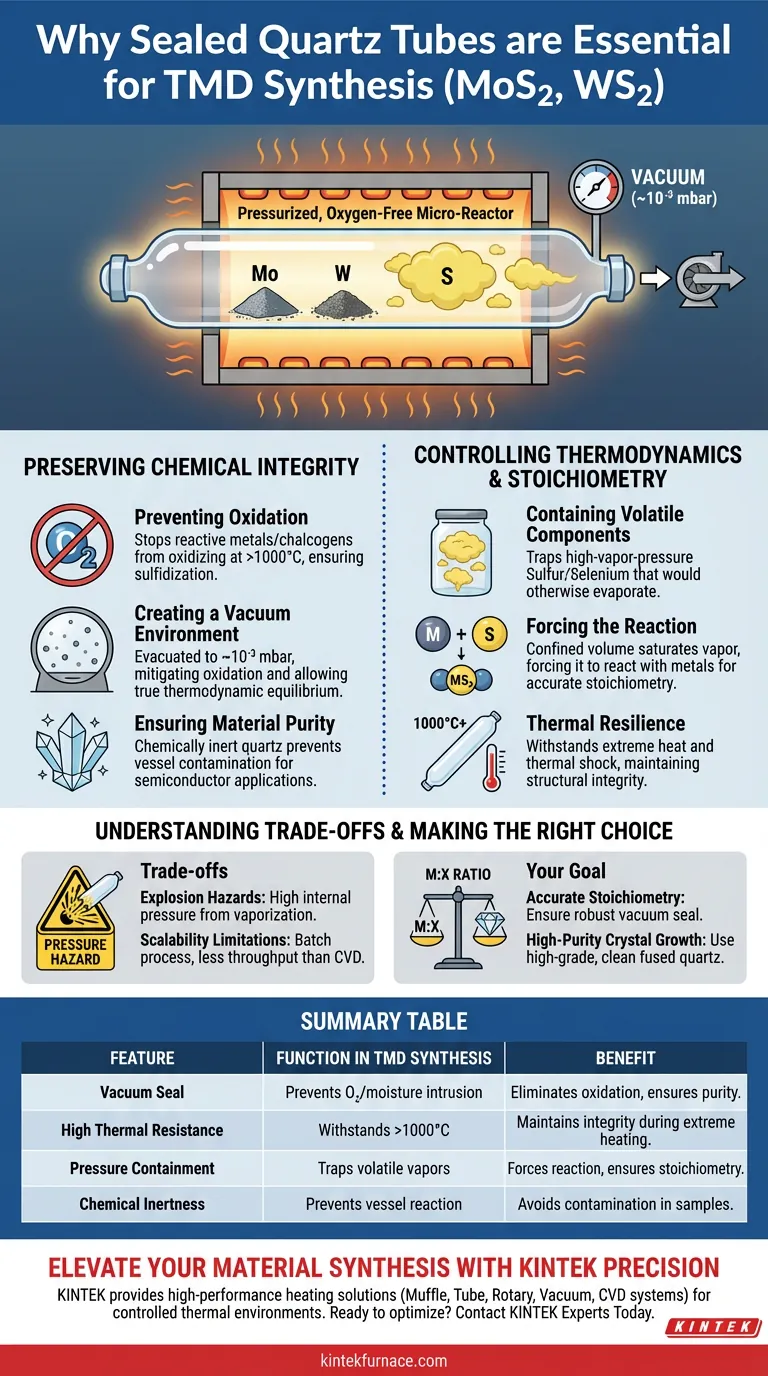
Sealed quartz tubes are strictly required for the synthesis of Transition Metal Dichalcogenides (TMDs) to create a pressurized, oxygen-free micro-environment capable of withstanding extreme heat. They serve the dual purpose of isolating reactive materials from atmospheric oxidation and physically containing volatile elements like sulfur to ensure the correct chemical reaction occurs.
The synthesis of MoS2 and WS2 relies on precise stoichiometry and extreme purity. A sealed quartz vessel acts as a closed micro-reactor that maintains a specific vacuum pressure while preventing the evaporation of essential reagents and the intrusion of contaminants.

Preserving Chemical Integrity
Preventing Oxidation
At reaction temperatures often exceeding 1000 °C, transition metals and chalcogens are highly reactive to oxygen. Even trace amounts of air will cause the raw materials to oxidize rather than sulfidize, ruining the sample.
Creating a Vacuum Environment
To mitigate oxidation, the quartz tube is evacuated to a high vacuum, typically around 10⁻³ mbar. This creates a pristine environment where the phase boundary data reflects the true thermodynamic equilibrium of the alloy, not a reaction with atmospheric gases.
Ensuring Material Purity
High-purity fused quartz is chemically inert, preventing the vessel itself from reacting with the precursors. This isolation stops container impurities from infiltrating the sensitive growth environment, which is critical for semiconductor applications.
Controlling Thermodynamics and Stoichiometry
Containing Volatile Components
Sulfur and selenium have high vapor pressures and volatilize readily at synthesis temperatures. In an open system, these elements would evaporate and escape before reacting with the metal.
Forcing the Reaction
By sealing the tube, the vaporized sulfur is trapped within a confined volume. This saturation forces the sulfur vapor to react with the metal powders (such as Molybdenum or Tungsten), ensuring the final material maintains accurate stoichiometry.
Thermal Resilience
Quartz is one of the few materials transparent to light that can withstand the thermal shock and sustained heat of the process. It maintains structural integrity while allowing researchers to visually monitor the reaction state if necessary.
Understanding the Trade-offs
Explosion Hazards
Because the system is sealed, the vaporization of sulfur creates significant internal pressure. If the stoichiometry is miscalculated or the tube walls are compromised, the vessel can explode inside the furnace.
Scalability Limitations
Sealed tube synthesis is inherently a batch process. While excellent for high-quality crystal growth and phase analysis, it lacks the continuous throughput capabilities of flow-based Chemical Vapor Deposition (CVD) systems.
Making the Right Choice for Your Goal
To ensure successful synthesis, align your setup with your specific experimental needs:
- If your primary focus is accurate stoichiometry: Ensure the vacuum seal is robust ($10^{-3}$ mbar) to prevent sulfur loss, which guarantees the ratio of metal to chalcogen remains constant.
- If your primary focus is high-purity crystal growth: Use high-grade fused quartz and rigorously clean the tube to prevent cation exchange with impurities or residual moisture.
The sealed quartz tube is not just a container; it is an active component of the thermodynamic system that makes TMD synthesis physically possible.
Summary Table:
| Feature | Function in TMD Synthesis | Benefit |
|---|---|---|
| Vacuum Seal | Prevents atmospheric oxygen/moisture intrusion | Eliminates oxidation and ensures chemical purity |
| High Thermal Resistance | Withstands temperatures >1000°C | Maintains structural integrity during extreme heating |
| Pressure Containment | Traps volatile sulfur/selenium vapors | Forces reaction with metals to ensure stoichiometry |
| Chemical Inertness | Prevents reaction between vessel and precursors | Avoids contamination in semiconductor samples |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect stoichiometric balance for MoS2 and WS2 requires more than just a tube; it requires a controlled thermal environment. KINTEK provides the high-performance heating solutions necessary to drive these critical reactions.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which can be customized to meet your unique laboratory needs. Whether you are performing batch synthesis in sealed quartz or scaling up via CVD, our equipment ensures the temperature stability and vacuum integrity your research demands.
Ready to optimize your high-temperature processes?
Visual Guide
References
- Dipanshu Sharma, Jwo‐Huei Jou. Two-Dimensional Transition Metal Dichalcogenide: Synthesis, Characterization, and Application in Candlelight OLED. DOI: 10.3390/molecules30010027
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the purpose of maintaining a specific argon flow in a tube furnace? Optimize LFP/C Composite Synthesis
- What are the space and footprint considerations for vertical and horizontal tube furnaces? Optimize Your Lab Layout
- What is the orientation referred to by the term 'horizontal' in horizontal tube furnaces? Optimize Your Thermal Processing with Expert Insights
- How does a benchtop fixed-bed quartz reactor simulate industrial conditions? Evaluate Pt-Ni Catalyst Stability
- How do resistance heating tube furnaces generate heat? Master Precise Temperature Control
- Why is a tube furnace required for the calcination of TiO2 in an H2/Ar mixed atmosphere? Engineering TiO2-X Defects
- What role does a continuous bench-scale drop tube pyrolyzer play in FPBO? Maximize High-Quality Bio-oil Yields
- What is the function of a tube reduction furnace? Enhance Ru@PG Catalysts with Ar/H2 Precision



















