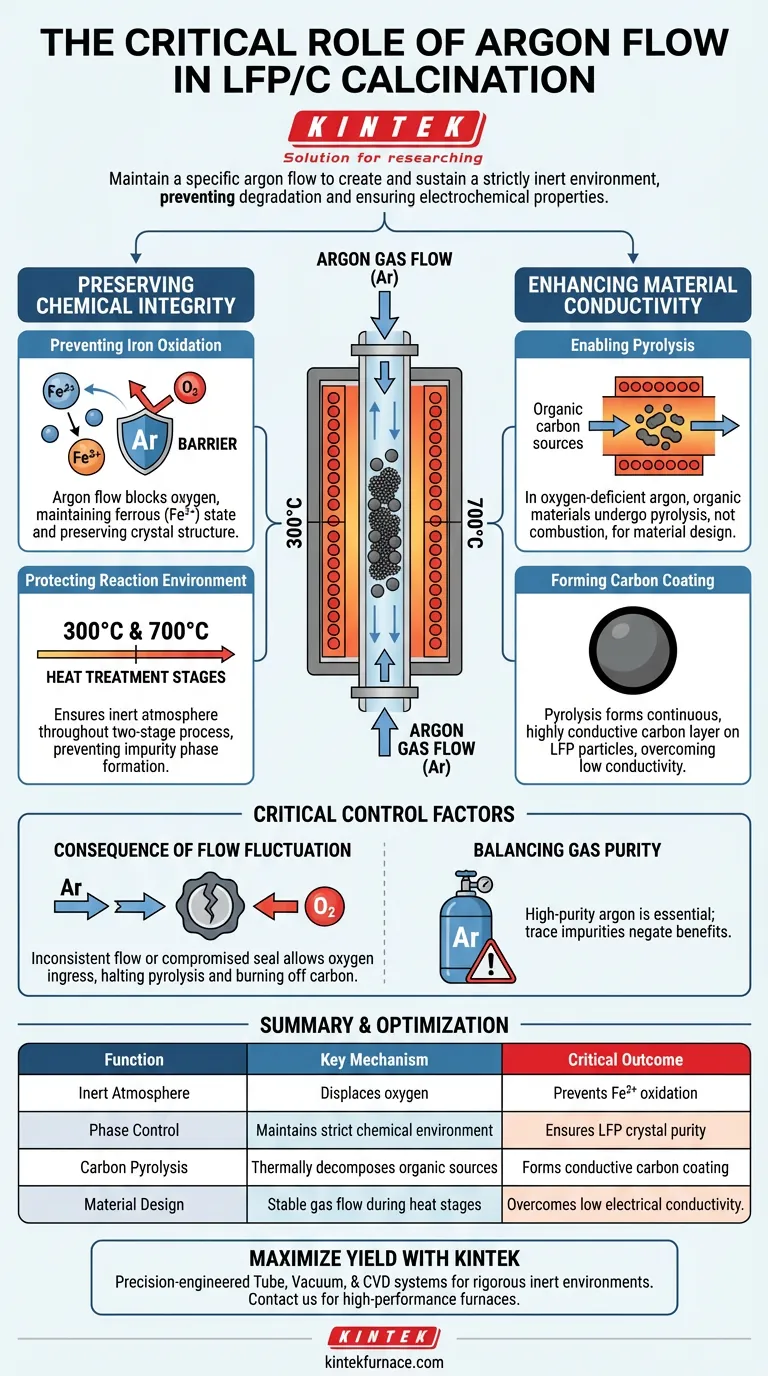
The primary purpose of maintaining a specific argon flow is to create and sustain a strictly inert environment. This flow displaces oxygen within the high-temperature tube furnace, preventing chemical degradation during the sensitive calcination of Lithium Iron Phosphate (LFP) composites. Without this controlled atmosphere, the synthesis process will fail to produce active cathode material with the necessary electrochemical properties.
The argon atmosphere performs a dual function: it prevents the oxidation of ferrous ions (Fe²⁺) into detrimental ferric ions (Fe³⁺) and enables the pyrolysis of organic sources into a uniform, conductive carbon coating.
Preserving Chemical Integrity
Preventing Iron Oxidation
The core stability of Lithium Iron Phosphate relies on iron maintaining a ferrous (Fe²⁺) valence state.
If oxygen is present in the furnace, these ions will oxidize into ferric ions (Fe³⁺). The argon flow acts as a barrier, strictly controlling oxygen content to ensure the correct crystal structure is preserved.
Protecting the Reaction Environment
This protection is critical throughout the entire two-stage heat treatment process, typically occurring at 300°C and 700°C.
Any breach in the inert atmosphere during these high-temperature phases can lead to the formation of impurity phases that degrade battery performance.
Enhancing Material Conductivity
Enabling Pyrolysis
The synthesis of LFP/C composites involves organic carbon sources that must be converted into elemental carbon.
In an oxygen-deficient argon environment, these organic materials undergo pyrolysis rather than combustion. This thermal decomposition is essential for the material design.
Forming the Carbon Coating
The result of this pyrolysis is a carbon layer that deposits directly onto the lithium iron phosphate particles.
A steady argon flow ensures this coating forms a continuous, uniform, and highly conductive layer. This layer is vital for overcoming the naturally low electrical conductivity of pure LFP.
Critical Control Factors
The Consequence of Flow Fluctuation
Inconsistent argon flow is a common source of batch failure.
If the flow drops or the furnace seal is compromised, oxygen ingress will immediately halt the pyrolysis process and burn off the carbon source. This leaves the cathode material without its conductive network and prone to oxidation.
Balancing Gas Purity
High-purity argon is non-negotiable for this process.
Even trace amounts of impurities in the gas supply can interfere with the reduction of the carbon source or react with the iron, negating the benefits of the inert environment.
Optimizing Your Calcination Strategy
To ensure high-performance LFP/C composites, align your furnace parameters with your specific material goals.
- If your primary focus is Phase Purity: Ensure the argon flow is established well before heating begins to completely purge oxygen and prevent Fe²⁺ oxidation.
- If your primary focus is Conductivity: Verify that the inert atmosphere is stable throughout the 700°C stage to maximize the uniformity of the carbon coating formed via pyrolysis.
Strict management of the argon atmosphere is the single most effective variable for controlling both the structural stability and electrical performance of your final composite.
Summary Table:
| Function | Key Mechanism | Critical Outcome |
|---|---|---|
| Inert Atmosphere | Displaces oxygen within the tube | Prevents Fe²⁺ oxidation to Fe³⁺ |
| Phase Control | Maintains strict chemical environment | Ensures purity of LFP crystal structure |
| Carbon Pyrolysis | Thermally decomposes organic sources | Forms uniform, conductive carbon coating |
| Material Design | Stable gas flow during heat stages | Overcomes LFP’s low electrical conductivity |
Maximize Your Battery Material Yield with KINTEK
Precision is non-negotiable when synthesizing high-performance LFP/C composites. Backed by expert R&D and world-class manufacturing, KINTEK provides high-precision Tube, Vacuum, and CVD systems designed to maintain the rigorous inert environments required for sensitive calcination processes.
Whether you need customizable gas flow controls or superior thermal uniformity, our high-temp furnaces are engineered to prevent oxidation and optimize carbon coating. Contact KINTEK today to discuss your unique laboratory needs and discover how our advanced heating solutions can bring reliability to your material research.
Visual Guide
References
- Xiukun Jiang, Huajun Tian. Effect of Heteroatom Doping on Electrochemical Properties of Olivine LiFePO4 Cathodes for High-Performance Lithium-Ion Batteries. DOI: 10.3390/ma17061299
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is a tube furnace used for Solid State Polycondensation? Master Molecular Weight Control in SSP
- What are the key factors affecting temperature control in split tube furnaces? Ensure Precision and Uniformity
- What is the primary function of a tube furnace in Leidenfrost experiments? Preheating with Precision & Protection
- What is the technical necessity of using a tube furnace in the synthesis of CoFe@HNCS? Master Co-Shell Nanostructures
- What are tube furnaces made of? Choose the Right Material for Your Thermal Process
- How do the nitrogen atmosphere and temperature control in a tube furnace affect carbonized lignin fiber quality?
- What is the purpose of purging a tube furnace with argon for tellurium reduction? Ensuring Safety and Purity
- What critical conditions do laboratory tube furnaces provide for VLS growth of ZnO nanowires? Master Nanoscale Synthesis



















