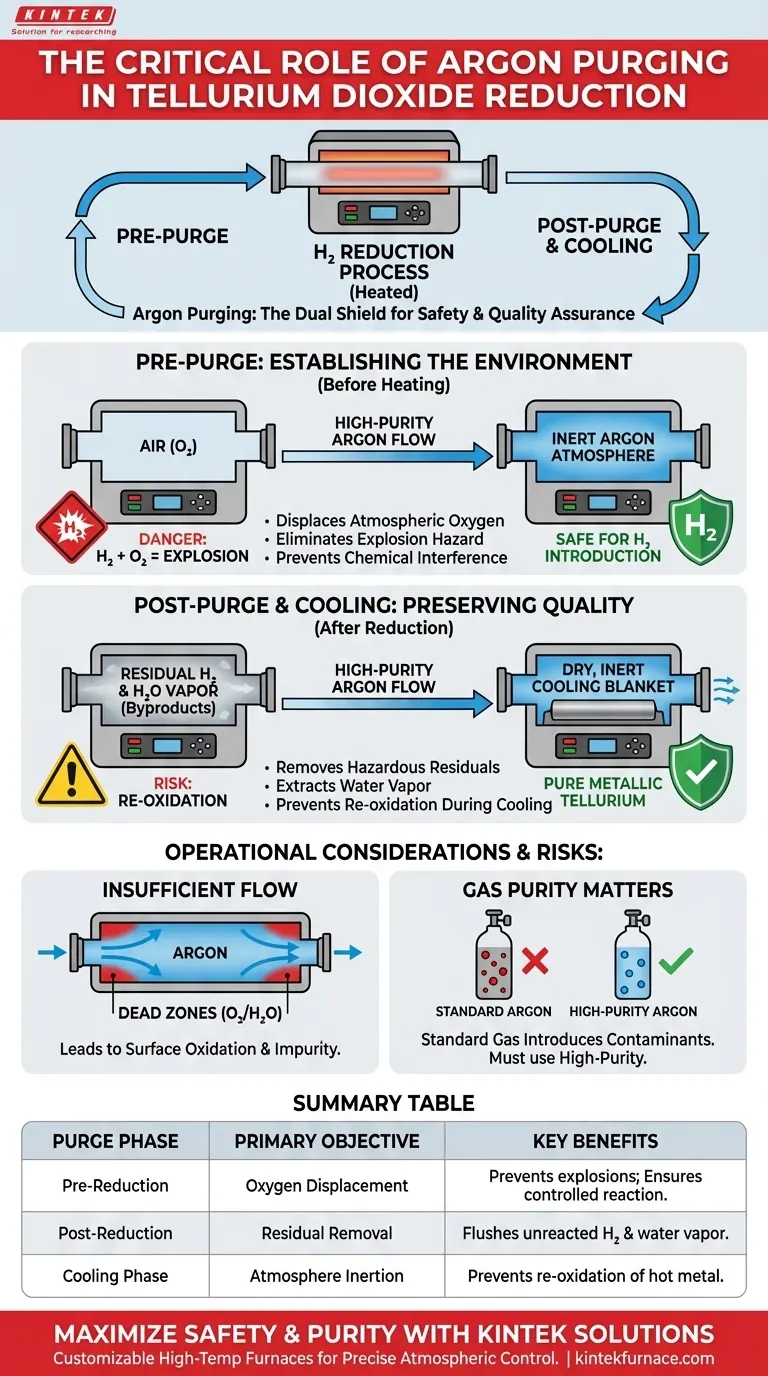
Purging a tube furnace with high-purity argon serves two critical, distinct functions depending on when it occurs in the production cycle. Before the process begins, it displaces atmospheric air to eliminate the risk of hydrogen-oxygen explosions and prevent interference with the reduction. After the process, it expels residual hydrogen and reaction byproducts to prevent the tellurium from re-oxidizing during the cooling phase.
The purging process acts as the primary safety shield and quality assurance step in hydrogen reduction. It ensures the environment is chemically inert before heating begins and remains stable while the product cools, preserving the purity of the metallic tellurium.

Establishing the Reaction Environment (Pre-Purge)
Preventing Catastrophic Failure
The most immediate danger in hydrogen reduction is the interaction between hydrogen gas and atmospheric oxygen. When these gases mix at the high temperatures required for reduction, they create an immediate explosion hazard.
Purging with argon before heating displaces the air inside the tube. This removes the oxygen fuel source, rendering the internal atmosphere safe for the introduction of hydrogen.
Eliminating Chemical Interference
Beyond safety, the presence of atmospheric oxygen compromises the chemical efficiency of the reduction. Oxygen acts as a variable that can interfere with the precise stoichiometry required to reduce tellurium dioxide.
By establishing an inert argon atmosphere, you ensure that the subsequent chemical reactions are driven solely by the controlled introduction of hydrogen.
Preserving Product Quality (Post-Purge)
Removing Hazardous Residuals
Once the reduction is complete, the furnace tube still contains unreacted hydrogen. This residual gas poses a safety risk if the system is opened immediately or if air enters while the system is hot.
A post-process argon purge flushes this remaining hydrogen out of the system. This effectively "resets" the atmosphere to a non-flammable state before the furnace is opened.
Extracting Water Vapor
The chemical reduction of tellurium dioxide generates water vapor as a byproduct. If this moisture remains in the tube during the cooling phase, it can negatively interact with the newly formed metal.
Argon flow carries this generated water vapor out of the reaction zone. This ensures the cooling environment is dry and chemically neutral.
Preventing Re-oxidation
Metallic tellurium is susceptible to oxidation, particularly while it is still hot. If the reduced metal is exposed to air or moisture before it has fully cooled, it will re-oxidize, undoing the reduction process.
Purging ensures the metal cools under a blanket of inert gas. This preserves the metallic state and guarantees the high purity of the final product.
Operational Considerations and Risks
The Consequence of Insufficient Flow
A common pitfall is purging for an insufficient duration or with inadequate flow rates. This can leave "dead zones" of oxygen or moisture within the tube, particularly near the ends.
Even trace amounts of trapped oxygen can lead to surface oxidation of the tellurium, resulting in a product that fails purity specifications.
The Necessity of "High-Purity" Gas
The effectiveness of this process is entirely dependent on the grade of argon used. Standard industrial argon often contains trace impurities, including oxygen and moisture.
Using anything less than high-purity argon introduces the exact contaminants you are trying to remove. This compromises the inert seal and can lead to inconsistent reduction results.
Maximizing Safety and Yield
To achieve a safe process and a high-purity product, you must view the purge as a critical reaction step, not just a preparation task.
- If your primary focus is Personnel Safety: Prioritize the pre-purge duration to ensure 100% volume displacement of oxygen before introducing hydrogen.
- If your primary focus is Product Purity: extend the post-purge phase until the furnace reaches room temperature to absolutely guarantee no re-oxidation occurs.
The integrity of your metallic tellurium relies as much on the inert gas discipline as it does on the reduction temperature itself.
Summary Table:
| Purge Phase | Primary Objective | Key Benefits |
|---|---|---|
| Pre-Reduction | Oxygen Displacement | Prevents hydrogen explosions and ensures a controlled reaction environment. |
| Post-Reduction | Residual Removal | Flushes unreacted hydrogen and water vapor byproducts from the tube. |
| Cooling Phase | Atmosphere Inertion | Prevents hot metallic tellurium from re-oxidizing upon contact with air. |
Maximize Your Lab’s Safety and Material Purity with KINTEK
Precise atmospheric control is the difference between a successful reduction and a catastrophic failure. At KINTEK, we understand the critical nature of gas discipline in high-temperature applications.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temp furnaces are fully customizable to meet your unique gas-purging requirements, ensuring high-purity results for tellurium reduction and beyond.
Ready to upgrade your thermal processing capabilities? Contact our technical specialists today to find the perfect furnace solution for your specific research or production needs.
Visual Guide
References
- Hanwen Chung, Bernd Friedrich. Hydrogen Reduction of Tellurium Oxide in a Rotary Kiln, Initial Approaches for a Sustainable Process. DOI: 10.3390/cryst15050478
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How does a fast Joule-heating device differ from a tubular furnace? Kinetic vs. Thermodynamic Control
- What are the advantages of horizontal tube furnaces? Achieve Superior Thermal Uniformity and Flexibility
- Why is high-purity quartz tube vacuum sealing required for Ag2S1-xTex? Protect Your Semiconductor Synthesis
- What is the function of an industrial-grade tube furnace? Mastering Expanded Graphite (EG) Calcination
- How does a tube furnace achieve precise control over product components? Master Cottonseed Pyrolysis with Precision
- Why is high-purity argon gas essential during the pyrolysis of Cu@Zn-NC in a high-temperature tube furnace?
- What types of atmospheres can be controlled in an atmosphere tube furnace? Master Precise Gas Environments for Material Processing
- What role does a laboratory tube furnace serve during Si/Al2O3/RGO synthesis? Precise Thermal Reduction & Bonding



















