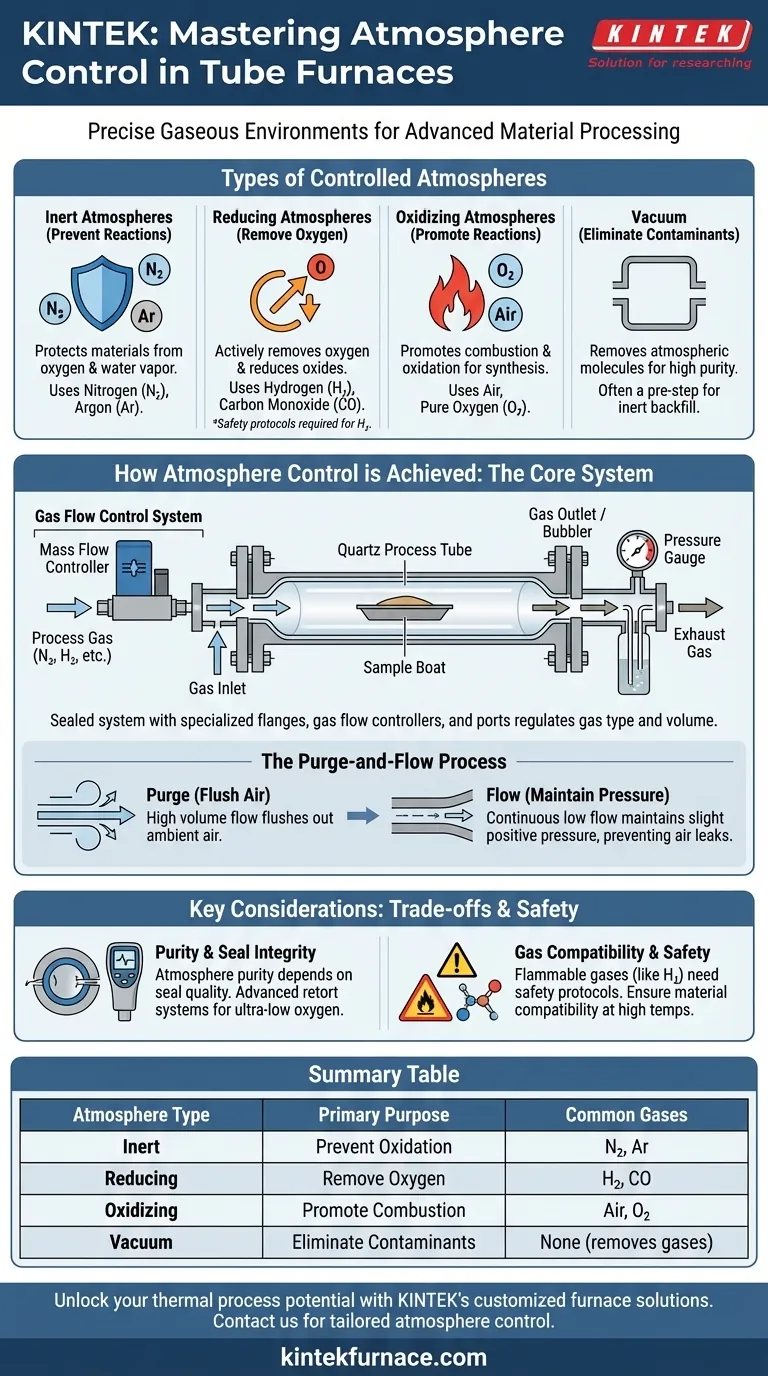
In short, an atmosphere tube furnace gives you precise control over a wide range of gaseous environments. These furnaces can operate with inert gases like nitrogen and argon, reducing gases like hydrogen, and oxidizing atmospheres like air or pure oxygen. Many models also support processing under a vacuum to remove atmospheric contaminants entirely.
The core function of an atmosphere tube furnace is not just to heat a sample, but to control the chemical environment in which it is heated. The choice of atmosphere is a critical process variable that determines whether you prevent, promote, or reverse specific chemical reactions.

The Purpose of Each Atmosphere Type
Understanding the goal of your process is the key to selecting the right atmosphere. Each category of gas serves a distinct chemical purpose.
Inert Atmospheres: Preventing Unwanted Reactions
The most common use of an atmosphere furnace is to create an inert environment. This is done to protect materials from reacting with oxygen or water vapor in ambient air at high temperatures.
Nitrogen (N2) is a cost-effective and widely used inert gas. Argon (Ar) is heavier than air and even more inert, making it ideal for extremely sensitive materials where even nitrogen could potentially react.
Reducing Atmospheres: Actively Removing Oxygen
A reducing atmosphere goes a step further than an inert one. It contains gases that actively react with and remove oxygen from the furnace chamber and, in some cases, from the material itself.
Hydrogen (H2) is a powerful reducing agent used to prevent and reverse oxidation on metals. Carbon Monoxide (CO) can also be used, often in specific chemical synthesis processes.
Oxidizing Atmospheres: Promoting Specific Reactions
Sometimes, the goal is to intentionally introduce oxygen to cause a reaction. An oxidizing atmosphere is used for processes like combustion, calcination, or certain types of material synthesis.
This can be achieved simply by flowing air through the tube or by introducing pure oxygen (O2) to increase the reaction rate and intensity.
Vacuum: The Ultimate Clean Environment
For the most sensitive materials, even trace amounts of gas can be problematic. Pulling a vacuum on the tube removes the vast majority of atmospheric molecules.
This is often a preliminary step before backfilling the tube with a high-purity inert gas, ensuring the starting environment is as clean as possible.
How Atmosphere Control is Achieved
The ability to manipulate the furnace environment relies on a combination of physical design and a controlled process.
The Core System Components
A tube furnace achieves atmospheric control through a sealed system. A quartz or ceramic process tube is sealed at both ends with specialized sealing flanges.
These flanges feature ports for a gas inlet, a gas outlet (or bubbler), and instrumentation like pressure gauges. A gas flow control system, often using mass flow controllers, precisely regulates the type and volume of gas entering the tube.
The Purge-and-Flow Process
Control is typically established in two steps. First, the chamber is purged by flowing a high volume of the desired gas to flush out all the ambient air.
Second, a continuous, low-volume flow of the gas is maintained throughout the heating process. This creates a slight positive pressure inside the tube, ensuring that any potential micro-leaks cause the process gas to flow out, not air to leak in.
Understanding the Trade-offs and Limitations
While powerful, atmosphere furnaces are not without their operational constraints. Achieving a perfect atmosphere requires understanding the system's limitations.
Purity and Seal Integrity
The purity of your atmosphere is directly dependent on the quality of your system's seals. Basic, economical furnace setups are excellent for most inert and reducing work but may struggle to achieve the ultra-low oxygen levels (measured by dew point) required for highly sensitive applications.
More advanced and costly systems, known as retort furnaces, use welded enclosures to provide a cleaner atmosphere but come with higher maintenance requirements.
Gas Compatibility and Safety
Not all gases can be used without special considerations. Flammable gases like hydrogen require robust safety protocols, leak detection, proper ventilation, and often specialized furnace components to prevent ignition.
Furthermore, some process gases can react with the furnace tube or heating elements at very high temperatures, requiring careful selection of materials for long-term reliability.
Choosing the Right Atmosphere for Your Goal
Your choice should be dictated entirely by the desired outcome of your thermal process.
- If your primary focus is preventing oxidation of a sensitive material: Use an inert gas like Argon or Nitrogen to create a protective environment.
- If your primary focus is actively removing oxides or creating an oxygen-free state: A reducing atmosphere containing Hydrogen is your most effective tool, with appropriate safety measures.
- If your primary focus is calcination or controlled material combustion: An oxidizing atmosphere using air or pure oxygen is necessary.
- If your primary focus is achieving the highest purity with no gas interaction: A vacuum system is required, either alone or before backfilling with an inert gas.
Ultimately, selecting the correct atmosphere transforms the tube furnace from a simple heater into a precision instrument for material engineering.
Summary Table:
| Atmosphere Type | Common Gases | Primary Purpose |
|---|---|---|
| Inert | Nitrogen, Argon | Prevent oxidation and unwanted reactions |
| Reducing | Hydrogen, Carbon Monoxide | Remove oxygen and reduce oxides |
| Oxidizing | Air, Pure Oxygen | Promote combustion and oxidation reactions |
| Vacuum | None (removes gases) | Eliminate contaminants for high-purity environments |
Unlock the full potential of your thermal processes with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored options like Tube Furnaces, Muffle Furnaces, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, enhancing efficiency and results. Contact us today to discuss how we can support your specific atmosphere control needs and drive innovation in your lab!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- How do vertical tube furnaces comply with environmental standards? A Guide to Clean, Efficient Operation



















