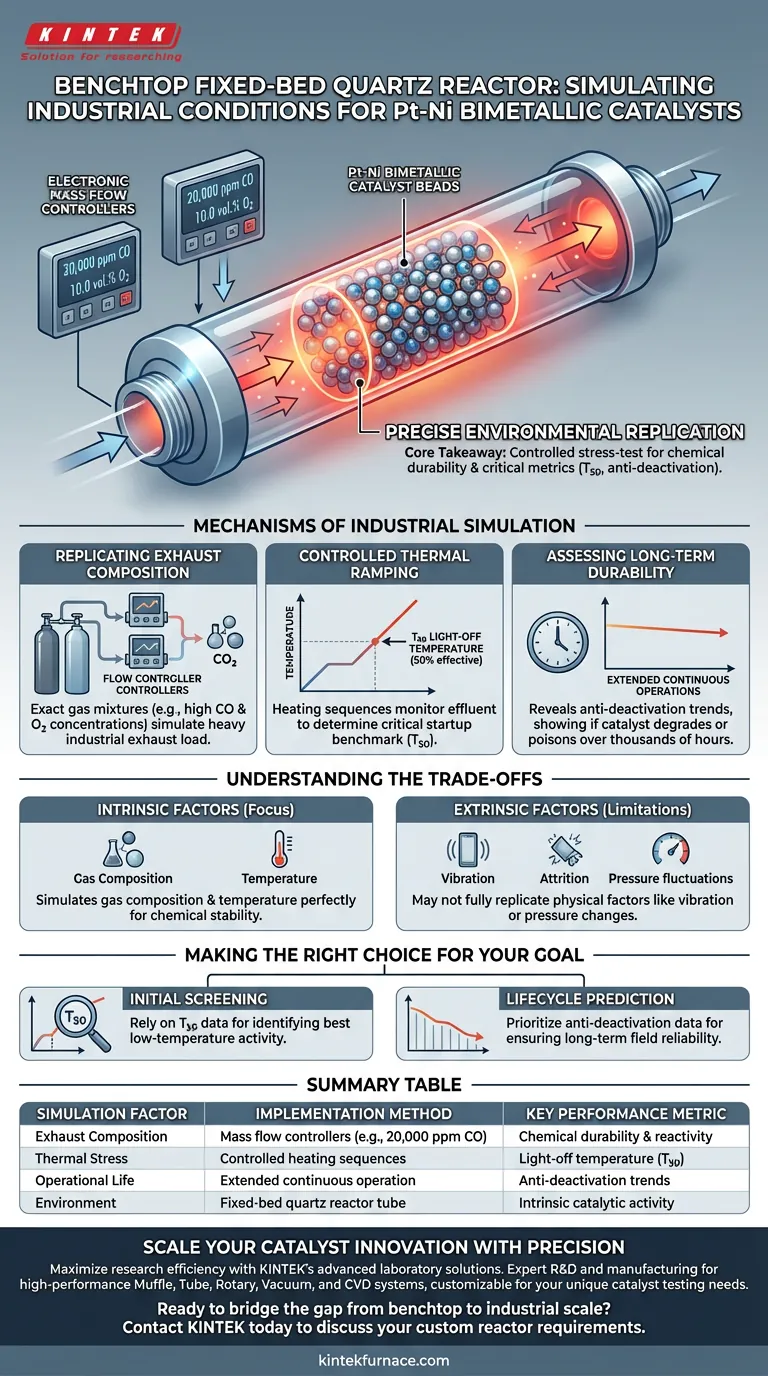
Precise environmental replication is the primary mechanism by which a benchtop fixed-bed quartz reactor simulates industrial conditions. By utilizing electronic mass flow controllers to deliver exact gas mixtures—such as 20,000 ppm CO and 10.0 vol.% O2—the system subjects Pt-Ni bimetallic catalysts to the specific chemical and thermal stresses found in actual industrial exhaust streams.
Core Takeaway The reactor functions as a controlled stress-test environment, isolating the catalyst's chemical durability by maintaining continuous, high-concentration gas flow during extended operation. This allows for the precise measurement of critical performance metrics, specifically light-off temperatures (T50) and anti-deactivation trends, before scaling up to full industrial trials.

Mechanisms of Industrial Simulation
Replicating Exhaust Composition
To evaluate a catalyst effectively, the test environment must mirror the chemical aggression of the real world. This setup uses electronic mass flow controllers to blend gases into precise recipes.
For Pt-Ni catalysts, this often involves high concentrations of Carbon Monoxide (CO) and Oxygen (O2). The system ensures these specific ratios (e.g., 20,000 ppm CO) are maintained strictly, simulating the heavy load of an industrial exhaust pipe.
Controlled Thermal Ramping
Stability is not just about handling gas; it is about handling heat while processing that gas. The reactor utilizes controlled heating sequences to monitor the reaction effluent.
This allows researchers to identify the light-off temperature (T50). This metric indicates the specific temperature at which the catalyst becomes 50% effective, a critical benchmark for industrial startup phases.
Assessing Long-Term Durability
Industrial catalysts must operate for thousands of hours, not just minutes. The fixed-bed reactor simulates this by running extended continuous operations.
By monitoring the effluent over long periods, the system reveals anti-deactivation performance. This exposes whether the Pt-Ni catalyst degrades, poisons, or loses efficiency over time under constant chemical attack.
Understanding the Trade-offs
Intrinsic vs. Extrinsic Factors
While this setup is excellent for determining chemical stability, it focuses on intrinsic catalytic activity. It simulates the gas composition and temperature of the industrial environment perfectly.
However, it is a "fixed-bed" simulation. It may not fully replicate physical industrial factors such as mechanical vibration, physical attrition, or erratic fluctuations in pressure that occur in large-scale plants.
Making the Right Choice for Your Goal
When interpreting data from a fixed-bed quartz reactor, consider your specific development stage:
- If your primary focus is Initial Screening: Rely on the T50 (light-off temperature) data to quickly identify which Pt-Ni ratios offer the best low-temperature activity.
- If your primary focus is Lifecycle Prediction: Prioritize the anti-deactivation performance data from extended continuous operation to ensure the catalyst won't fail prematurely in the field.
Use this benchtop simulation to validate the chemical robustness of your Pt-Ni catalyst before investing in expensive pilot-scale manufacturing.
Summary Table:
| Simulation Factor | Implementation Method | Key Performance Metric |
|---|---|---|
| Exhaust Composition | Mass flow controllers (e.g., 20,000 ppm CO) | Chemical durability & reactivity |
| Thermal Stress | Controlled heating sequences | Light-off temperature (T50) |
| Operational Life | Extended continuous operation | Anti-deactivation trends |
| Environment | Fixed-bed quartz reactor tube | Intrinsic catalytic activity |
Scale Your Catalyst Innovation with Precision
Maximize your research efficiency with KINTEK’s advanced laboratory solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique catalyst testing needs. Whether you are evaluating Pt-Ni bimetallic stability or simulating complex industrial exhaust streams, our equipment provides the thermal and chemical control necessary for reliable data.
Ready to bridge the gap from benchtop to industrial scale? Contact KINTEK today to discuss your custom reactor requirements.
Visual Guide
References
- Min Xu, John T. S. Irvine. Synergistic growth of nickel and platinum nanoparticles via exsolution and surface reaction. DOI: 10.1038/s41467-024-48455-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the technical considerations for using quartz tubes for sulfidation? Optimize Your Material Purity & Stability
- What are the key features of a vacuum tube furnace? Master High-Temp Processing with Precision Control
- What is a three zone furnace? The Key to Superior Temperature Uniformity
- What is the primary function of a single-zone tube furnace for MoS2? Optimize Sulfidation with Precise Thermal Control
- How should a quartz tube furnace be cleaned? Essential Steps for Safe, Contamination-Free Maintenance
- What core environmental conditions does a laboratory tube furnace provide for MoS2 sulfurization? Master 750 °C Synthesis
- What are the thermal performance advantages of vacuum tube furnaces? Achieve Faster, Purer Heat Treatment
- What is the academic use of drop tube furnaces? Unlock Precise High-Temp Research for Materials and Energy



















