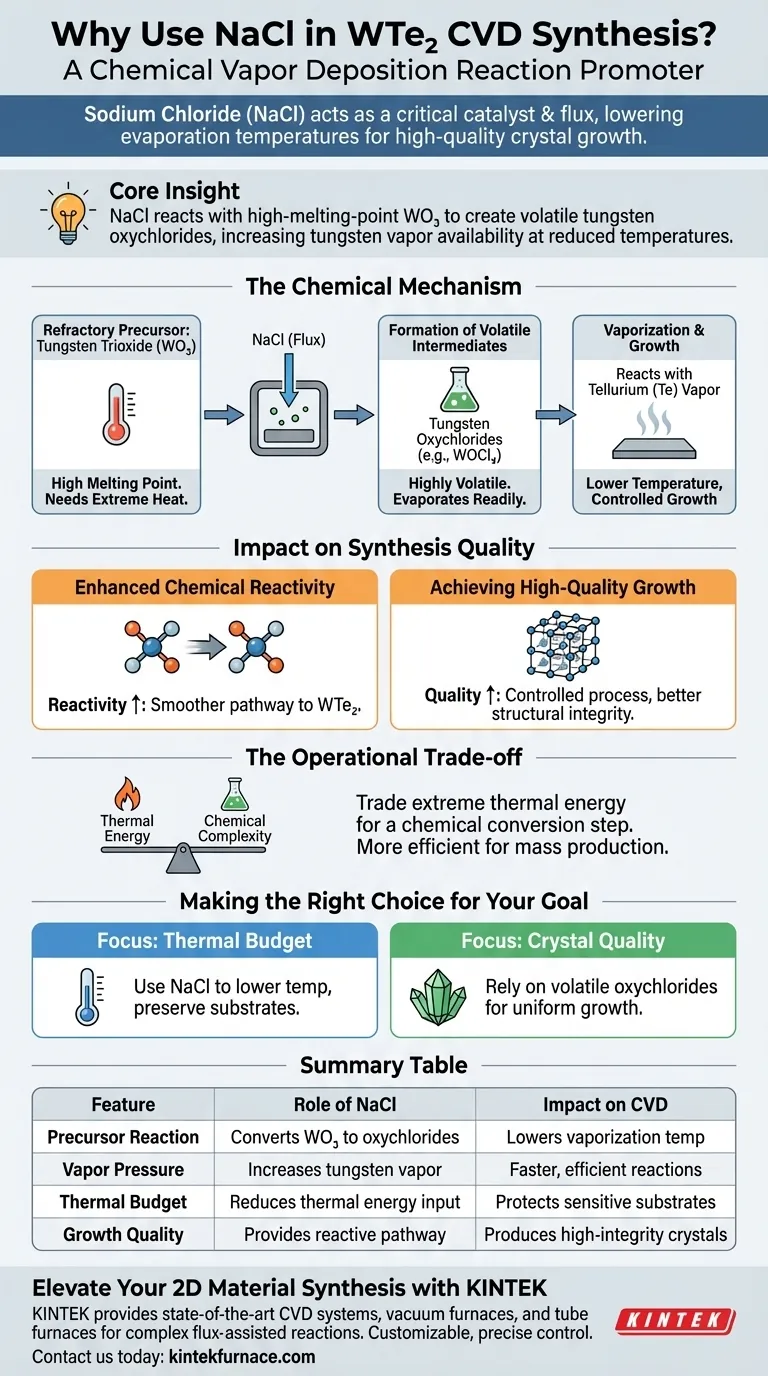
Sodium chloride (NaCl) serves as a critical reaction promoter that acts as both a catalyst and a flux during the synthesis of tungsten ditelluride (WTe2). By chemically transforming the tungsten source, it significantly lowers the evaporation temperature required for the process, enabling the growth of high-quality crystals without the need for excessive heat.
Core Insight: The primary function of NaCl is to react with the high-melting-point precursor, tungsten trioxide (WO3), to create volatile tungsten oxychlorides. These intermediates vaporize easily, increasing the availability of tungsten vapor to react with tellurium at reduced temperatures.

The Chemical Mechanism
The synthesis of tungsten ditelluride via Chemical Vapor Deposition (CVD) relies on mobilizing tungsten, a metal with naturally high thermal stability. NaCl facilitates this through a specific chemical pathway.
Converting Refractory Precursors
Tungsten trioxide (WO3) is commonly used as a source material, but it possesses a very high melting point.
Without an additive, vaporizing WO3 requires extremely high temperatures that can be impractical or detrimental to the substrate.
Formation of Volatile Intermediates
When NaCl is introduced, it reacts directly with the WO3.
This reaction produces tungsten oxychlorides, specifically compounds like WOCl2 or WOCl4.
Unlike the original oxide, these chloride-based intermediates are highly volatile and evaporate readily.
Impact on Synthesis Quality
The introduction of NaCl does more than just lower the vaporization temperature; it fundamentally changes the growth environment of the crystal.
Enhancing Chemical Reactivity
The tungsten oxychloride intermediates are far more reactive than pure tungsten oxide.
This heightened reactivity facilitates a more efficient combination with tellurium vapor.
The result is a smoother chemical pathway to forming the final tungsten ditelluride (WTe2) compound.
Achieving High-Quality Growth
By allowing the reaction to occur at lower temperatures, the process becomes more controlled.
This thermal reduction minimizes the chaotic growth often associated with extreme heat.
Consequently, the process yields high-quality WTe2 crystals with better structural integrity.
The Operational Trade-off
While CVD is generally praised for creating dense, uniform films and coating complex shapes, the use of a salt flux addresses a specific limitation regarding material properties.
Overcoming Thermal Constraints
The central trade-off involves the management of thermal energy versus chemical complexity.
Standard CVD of refractory metals typically demands high energy inputs to achieve vaporization.
By using NaCl, you trade the need for extreme thermal energy for a chemical conversion step, making the process more efficient and suitable for mass production contexts where lower temperatures are desirable.
Making the Right Choice for Your Goal
To determine how to best utilize this flux-assisted method, consider your specific synthesis objectives.
- If your primary focus is Thermal Budget: Utilize NaCl to lower the required vaporization temperature of the tungsten source, preserving sensitive substrates.
- If your primary focus is Crystal Quality: Rely on the formation of volatile oxychlorides to ensure a steady, reactive supply of tungsten for uniform growth.
NaCl transforms a high-energy thermal challenge into a manageable chemical reaction, unlocking the efficient production of high-quality 2D materials.
Summary Table:
| Feature | Role of NaCl in WTe2 Synthesis | Impact on CVD Process |
|---|---|---|
| Precursor Reaction | Converts WO3 into volatile tungsten oxychlorides | Lowers required vaporization temperature |
| Vapor Pressure | Increases availability of tungsten vapor | Enables faster and more efficient reactions |
| Thermal Budget | Reduces thermal energy input requirements | Protects sensitive substrates from extreme heat |
| Growth Quality | Provides a steady, reactive chemical pathway | Produces high-quality crystals with better integrity |
Elevate Your 2D Material Synthesis with KINTEK
Precision in Chemical Vapor Deposition requires more than just the right precursors—it demands the right environment. KINTEK provides state-of-the-art CVD systems, vacuum furnaces, and tube furnaces specifically designed to handle complex flux-assisted reactions like WTe2 synthesis.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet your unique laboratory needs, ensuring uniform heating and precise control over volatile intermediates. Ready to optimize your thin-film production? Contact KINTEK today to discover how our high-temperature solutions can drive your research forward.
Visual Guide
References
- Andrejs Terehovs, Gunta Kunakova. Chemical Vapor Deposition for the Fabrication of WTe<sub>2</sub>/h‐BN Heterostructures. DOI: 10.1002/admi.202500091
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the core logic of using vacuum coating for energy equipment? Boost Wear and Heat Resistance Effectively
- What is the function of a radio frequency (RF) plasma sputtering system? Precision PtNP Underlayer Fabrication
- How does Chemical Vapor Deposition (CVD) work? Master Thin Film Fabrication for Superior Materials
- What process conditions does CVI equipment provide for optimizing ceramic honeycombs? Enhance Microwave Absorption
- Why is CVT preferred over solid-phase reaction for Janus RhSeCl? Key Advantages in Crystal Growth
- What is the purpose of high-purity argon in Bi2Se3 CVD? Ensure High-Quality Film Growth with Optimal Carrier Gas
- How does CVD achieve high-purity and uniform films? Master Precise Film Deposition for Superior Results
- Why is mica preferred as a substrate for CVD growth of Mn3O4 nanosheets? Key Structural Advantages



















