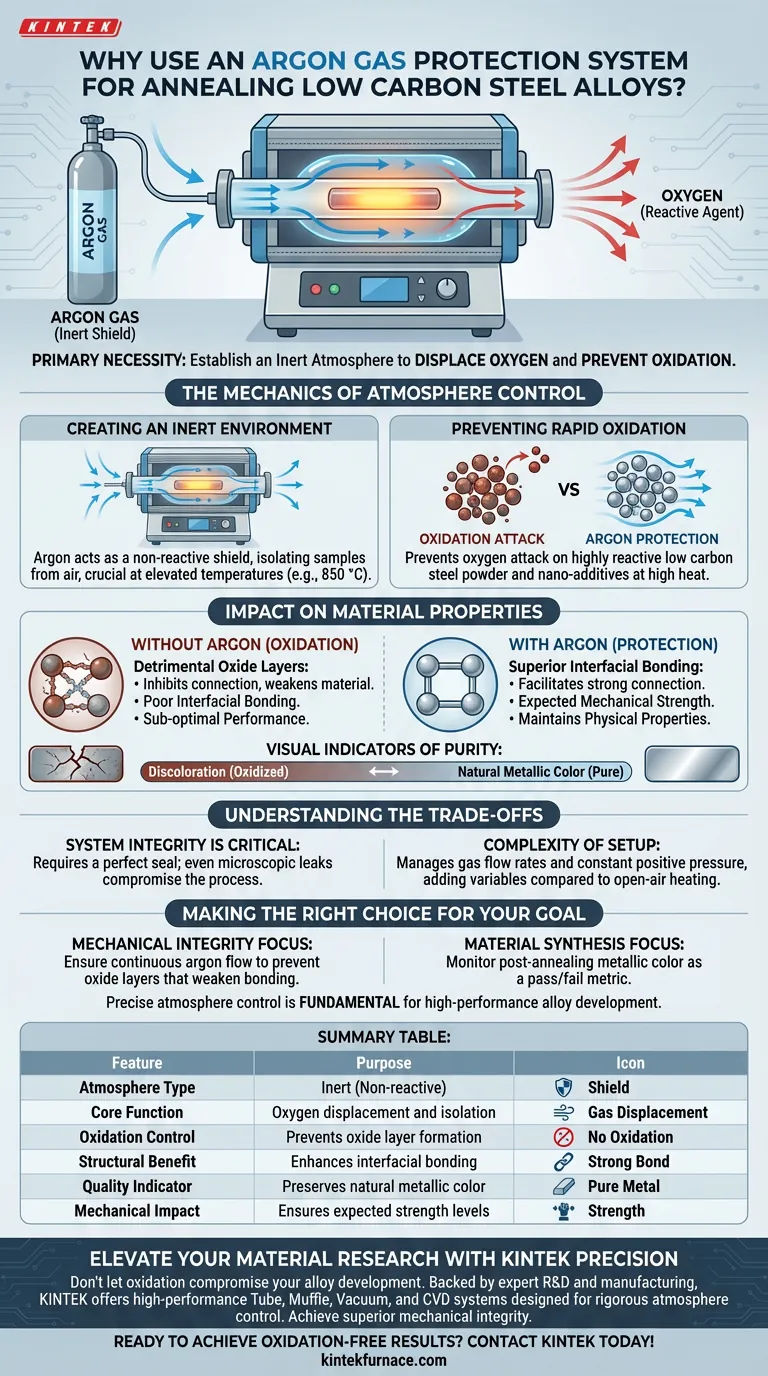
The primary necessity for using an argon gas protection system is to establish an inert atmosphere that completely isolates the low carbon steel samples from oxygen. Without this protective barrier during high-temperature annealing, the material will inevitably undergo oxidation, compromising both its surface chemistry and structural integrity.
By displacing oxygen within the furnace, argon gas prevents the formation of detrimental oxide layers on particle surfaces. This preservation is essential for achieving strong interfacial bonding and ensuring the material reaches its expected mechanical strength.

The Mechanics of Atmosphere Control
Creating an Inert Environment
Argon serves as a non-reactive shield, effectively displacing the air naturally present inside the laboratory tube furnace. This isolation is strictly required when subjecting materials to elevated temperatures, such as 850 °C.
Preventing Rapid Oxidation
At these high temperatures, low carbon steel—specifically metal powders and nano-additives—becomes highly reactive. Without a protective gas, oxygen attacks the material, causing rapid oxidation. Argon prevents this reaction from initiating.
Impact on Material Properties
Ensuring Superior Interfacial Bonding
For an alloy to possess structural integrity, its internal components must bond tightly at the microscopic level. Oxide layers formed during heating act as barriers that inhibit this connection. By preventing these layers, argon facilitates superior interfacial bonding between the alloy components.
Achieving Expected Mechanical Strength
The presence of oxides introduces weak points within the material, leading to sub-optimal performance. Using an argon protection system ensures the alloy maintains its intended physical properties and achieves the expected mechanical strength.
Visual Indicators of Purity
A key indicator of a successful annealing process is the visual appearance of the sample. Argon protection maintains the natural metallic color of the steel, whereas discoloration signals that oxidation has occurred and the material's purity is compromised.
Understanding the Trade-offs
System Integrity is Critical
The effectiveness of the process relies entirely on the furnace's ability to maintain a seal. Even a microscopic leak in the protection system allows oxygen to enter, which can ruin the sample surface despite the presence of argon.
Complexity of Setup
Compared to open-air heating, using an argon system adds variables to your experiment. You must manage gas flow rates and ensure constant positive pressure to prevent backflow of atmospheric air.
Making the Right Choice for Your Goal
To ensure the success of your annealing process, align your setup with your specific research objectives:
- If your primary focus is mechanical integrity: Ensure the argon flow is continuous to prevent oxide layers that weaken the bonding between metal powders.
- If your primary focus is material synthesis: Monitor the sample for metallic color post-annealing as a pass/fail metric for your atmosphere control system.
Precise atmosphere control is not merely a precautionary step; it is a fundamental requirement for developing high-performance alloy materials.
Summary Table:
| Feature | Purpose of Argon Protection in Annealing |
|---|---|
| Atmosphere Type | Inert (Non-reactive) |
| Core Function | Oxygen displacement and isolation |
| Oxidation Control | Prevents formation of detrimental oxide layers |
| Structural Benefit | Enhances interfacial bonding between particles |
| Quality Indicator | Preserves natural metallic color and purity |
| Mechanical Impact | Ensures the alloy reaches expected strength levels |
Elevate Your Material Research with KINTEK Precision
Don't let oxidation compromise your alloy development. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems designed to maintain the rigorous atmosphere control your research demands. Whether you need standard or fully customizable laboratory high-temp furnaces, our equipment ensures superior mechanical integrity for every sample.
Ready to achieve oxidation-free results? Contact KINTEK today to discuss your unique needs!
Visual Guide
References
- Abbas Ali Diwan, Mohammed J. Alshukri. Characterization of the mechanical properties for mild steel alloyed reinforcement with nanomaterials using powder technology. DOI: 10.1007/s43939-025-00280-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a high-temperature tube furnace necessary for carbonization of biochar? Unlock High-Performance Electrodes
- How is the temperature controlled in a tube furnace? Achieve Precise Thermal Processing for Your Lab
- What is the temperature accuracy of a three-zone split tube furnace? Achieve ±1°C Precision and Superior Uniformity
- How does a high-temperature tube atmosphere furnace contribute to nitrogen-doping of graphene oxide? Enhance Your R&D
- What critical environmental conditions does a tube furnace provide for volcanic rock thermal cycling? Expert Guide
- What process conditions are provided by a horizontal tube furnace for AuNPs@MOF catalysts? Precise Thermal Control
- What Role Does a Tube Reactor Play in Food Waste Pyrolysis? Control Carbonization for High-Quality Biochar
- What is the core function of a high-temperature tube furnace in converting Fe2O3/GO? Mastering Material Transformation



















