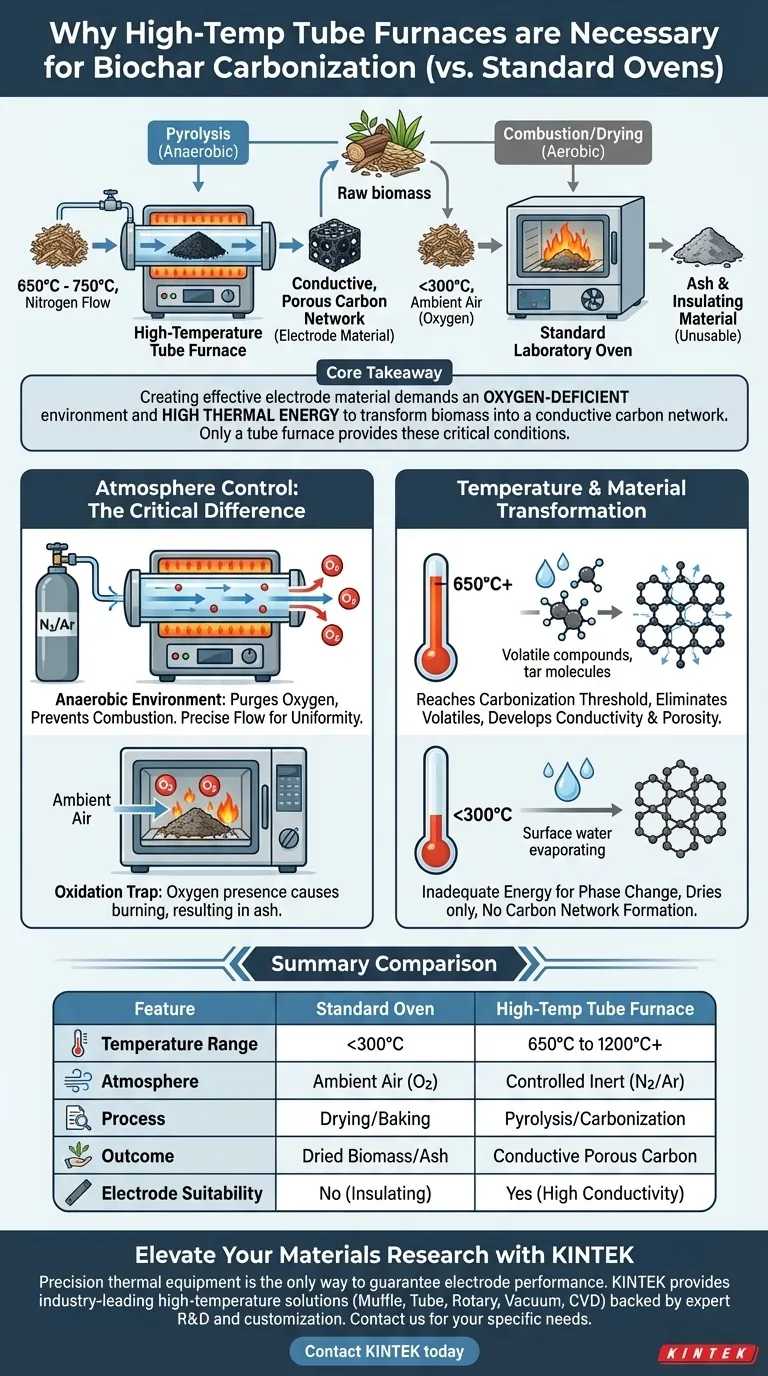
A high-temperature tube furnace is strictly required for carbonizing biochar electrode materials because it provides two critical conditions that standard ovens cannot: extreme temperatures and a controlled inert atmosphere. While a standard oven is designed for drying or baking in air, a tube furnace facilitates pyrolysis—the chemical decomposition of organic material—by maintaining temperatures between 650°C and 750°C under a constant flow of nitrogen to prevent combustion.
Core Takeaway Creating effective electrode material requires transforming raw biomass into a conductive, porous carbon network, a process that demands an oxygen-deficient environment and high thermal energy. A tube furnace creates these specific conditions to strip away volatile compounds without burning the material, whereas a standard oven would simply incinerate the biomass into ash or fail to trigger the necessary chemical changes.

The Critical Role of Atmosphere Control
Creating an Anaerobic Environment
The defining feature of a tube furnace is its ability to maintain a strictly controlled anaerobic environment. By constantly flowing an inert gas, such as nitrogen, through the tube, the furnace purges oxygen from the chamber.
Preventing Combustion
If you attempted to heat biomass to high temperatures in a standard oven, the presence of oxygen would cause the material to catch fire and burn away. The inert atmosphere of the tube furnace ensures the biomass undergoes thermal decomposition rather than combustion.
Precise Flow Control
Tube furnaces allow for a stable nitrogen flow field. This stability is crucial for ensuring uniform treatment of the material, preventing localized oxidation that could degrade the structural integrity of the final product.
Temperature Capabilities and Material Transformation
Reaching the Carbonization Threshold
Carbonization requires sustained temperatures ranging from 650°C to 750°C. Standard laboratory ovens typically max out at much lower temperatures (often around 250°C to 300°C), which are insufficient for converting biomass into elemental carbon.
Eliminating Volatiles
At these high temperatures, the furnace efficiently drives off volatile components (such as moisture and tars) found in the lignocellulosic biomass. Removing these non-carbon elements is essential to leave behind a pure carbon skeleton.
Developing Conductivity and Porosity
The removal of volatiles in this high-heat, oxygen-free environment creates a developed porous carbon network. This structure is what gives the biochar its high electrical conductivity and surface area, both of which are non-negotiable requirements for high-performance electrode materials.
Understanding the Trade-offs: Why Ovens Fail
Inadequate Energy for Phase Change
A standard oven can dry biomass, removing surface water. However, it lacks the thermal power to break the chemical bonds necessary to transform the internal structure of the material from wood/plant matter into graphitic or amorphous carbon.
The Oxidation Trap
Because standard ovens are not sealed against the atmosphere, they introduce oxygen during the heating process. This leads to the formation of insulating ash rather than conductive carbon, rendering the material useless for electronic applications.
Making the Right Choice for Your Goal
To ensure you select the correct equipment for your specific stage of research, consider the following:
- If your primary focus is pre-processing or drying: A standard oven is sufficient for removing moisture from raw biomass at temperatures below 110°C.
- If your primary focus is synthesizing electrode material: A high-temperature tube furnace is mandatory to achieve the 650°C+ temperatures and inert nitrogen atmosphere required for pyrolysis and conductivity.
Precision in your thermal equipment is the only way to guarantee the structural integrity and electrochemical performance of your biochar.
Summary Table:
| Feature | Standard Laboratory Oven | High-Temperature Tube Furnace |
|---|---|---|
| Temperature Range | Typically < 300°C | 650°C to 1200°C+ |
| Atmosphere Control | Ambient Air (Oxygen present) | Controlled Inert (Nitrogen/Argon) |
| Primary Process | Drying and Baking | Pyrolysis and Carbonization |
| Material Outcome | Dried Biomass or Ash | Conductive Porous Carbon |
| Electrode Suitability | No (Insulating properties) | Yes (High conductivity) |
Elevate Your Materials Research with KINTEK
Don't let inadequate thermal equipment compromise your electrode performance. KINTEK provides industry-leading high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all backed by expert R&D and precision manufacturing. Whether you need standard specifications or a fully customized furnace for unique carbonization requirements, our team is ready to deliver the reliability your lab demands.
Contact KINTEK today to discuss your customization needs
Visual Guide
References
- Geming Wang, Qirui Wu. Exploring a Porous Biochar-Based Capacitive Deionization Device for Phosphogypsum Wastewater Treatment in Undergraduate Experimental Teaching: Understanding, Development, and Practice. DOI: 10.1021/acsomega.5c05966
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the necessity of annealing treatment for CuCo2O4@rGO? Optimize High-Crystallinity Synthesis in Tube Furnaces
- Why is a tube reduction furnace used for the pre-reduction of CeAlOx/NiO/Ni-foam catalysts? Essential Catalyst Prep
- What are the unique features of a multi station vacuum tube furnace regarding atmosphere control? Unlock High-Purity Parallel Experiments
- What is the role of a laboratory tube furnace in the carbonization of peanut shells? Master Biochar Preparation
- Why is the 70mm tube furnace considered versatile? Ideal for High-Temp, Controlled-Atmosphere Lab Work
- What is a split tube furnace and what makes it versatile? Unlock Easy Access and Flexibility for Your Lab
- What specific process conditions does a laboratory tube furnace provide? Optimize Biomass Carbonization Success
- What core physical conditions does a tube furnace provide in the two-step synthesis of WS2? Master Film Growth



















