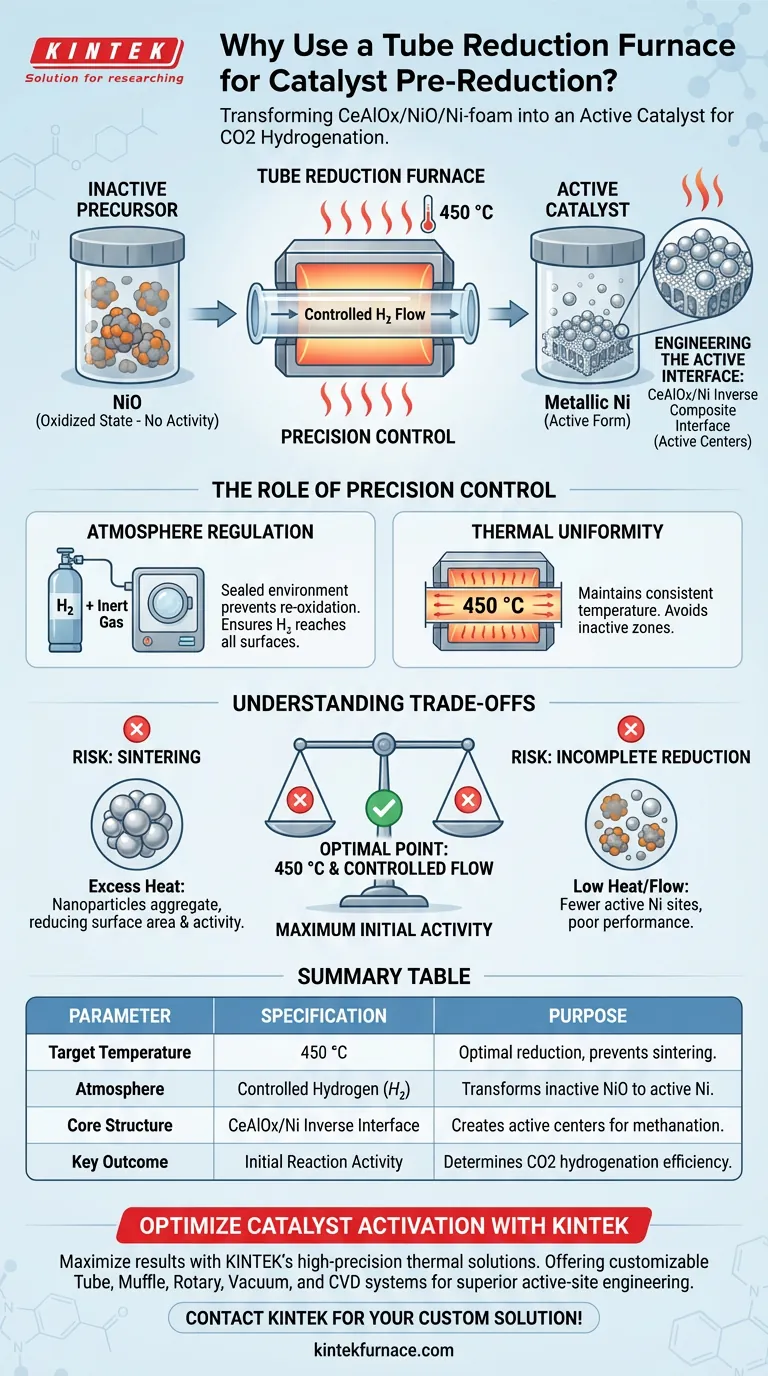
A tube reduction furnace is utilized to transform the catalyst from its synthesized, inactive oxidized state (NiO) into its active metallic form. By subjecting the CeAlOx/NiO/Ni-foam precursor to a controlled hydrogen flow at 450 °C, the furnace reduces Nickel oxides to metallic Nickel nanoparticles, creating the specific chemical interface required for CO2 hydrogenation.
The core function of this process is not merely chemical reduction, but the precise engineering of the "CeAlOx/Ni inverse composite interface." This structural arrangement, achieved only through controlled thermal treatment, generates the active centers that determine the catalyst's initial methanation activity and overall performance.

From Inactive Precursor to Active Catalyst
The Necessity of Chemical Reduction
Catalysts like CeAlOx/NiO/Ni-foam are typically synthesized in an oxidized state, specifically as Nickel Oxide (NiO).
NiO itself possesses no hydrogenation activity.
To trigger the reaction capabilities, the furnace uses a hydrogen atmosphere to strip oxygen atoms from the lattice, converting the material into metallic Nickel (Ni).
Constructing the Inverse Interface
The reduction process does more than simply create metal; it creates a specific microstructure.
The treatment builds a CeAlOx/Ni inverse composite interface.
This involves metallic Ni nanoparticles coming into intimate contact with the supporting oxides, forming the efficient active centers necessary for the methanation reaction.
Defining Initial Activity
The success of the CO2 hydrogenation process is directly linked to this pre-reduction step.
The quality of the reduction determines the density and nature of the active sites.
Consequently, the furnace treatment directly establishes the initial reaction activity of the catalyst.
The Role of Precision Control
Regulating the Atmosphere
A tube furnace is essential because it provides a sealed, controllable environment for hazardous or volatile gases.
It allows for the precise introduction of hydrogen gas (often mixed with inert gases like Nitrogen or Argon) to ensure a stable reductive atmosphere.
This prevents re-oxidation and ensures the reducing agent reaches all surfaces of the porous Ni-foam support.
Thermal Precision
The primary reference specifies a reduction temperature of 450 °C.
The tube furnace maintains this temperature with high uniformity, ensuring the reduction is consistent across the entire catalyst volume.
Without this thermal stability, parts of the catalyst might remain oxidized (inactive) while others could degrade.
Understanding the Trade-offs
The Risk of Sintering
While high heat is necessary for reduction, excessive heat or uncontrolled heating rates can be detrimental.
If the furnace temperature overshoots or dwells too long, the metallic nanoparticles may aggregate or "sinter."
Larger particles have less surface area, which significantly reduces the catalytic activity achieved during the process.
Incomplete Reduction
Conversely, if the temperature is too low or hydrogen flow is insufficient, the reduction from NiO to Ni will be incomplete.
This leaves the catalyst with fewer active metallic sites.
The result is a failure to form the critical CeAlOx/Ni interface, leading to poor performance in CO2 hydrogenation.
Making the Right Choice for Your Goal
To maximize the efficacy of your CeAlOx/NiO/Ni-foam catalyst, consider the following parameters during furnace operation:
- If your primary focus is Maximum Initial Activity: Ensure the furnace is calibrated to hold exactly 450 °C; deviations can alter the formation of the critical inverse composite interface.
- If your primary focus is Microstructural Uniformity: Prioritize the control of gas flow rates to ensure the hydrogen atmosphere is evenly distributed through the Ni-foam structure.
Ultimately, the tube reduction furnace is not just a heating device; it is the tool that architecturally defines the active sites of your catalyst.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Target Temperature | 450 °C | Optimal reduction without nanoparticle sintering |
| Atmosphere | Controlled Hydrogen ($H_2$) | Transforms inactive NiO to active metallic Nickel |
| Core Structure | CeAlOx/Ni Inverse Interface | Creates the active centers for methanation activity |
| Key Outcome | Initial Reaction Activity | Determines the efficiency of CO2 hydrogenation |
Optimize Your Catalyst Activation with KINTEK
Maximize your CO2 hydrogenation results with KINTEK’s high-precision thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs. Whether you are developing complex inverse composite interfaces or scaling up catalyst production, our systems provide the thermal stability and atmosphere control required for superior active-site engineering.
Contact KINTEK today to discuss your customized furnace solution!
Visual Guide
References
- Xin Tang, Lili Lin. Thermally stable Ni foam-supported inverse CeAlOx/Ni ensemble as an active structured catalyst for CO2 hydrogenation to methane. DOI: 10.1038/s41467-024-47403-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a single-zone tube furnace play in the synthesis of (100)-oriented MoO2 nanobelts? Precision APCVD Control
- What is the significance of the gas path control system in a laboratory tube furnace during activated carbon synthesis?
- How does a tube atmosphere furnace facilitate local CVD during PAN fiber carbonization? Master In-Situ CNT Growth
- Why is a vacuum tube furnace required for (Si/graphite/graphene)@C composite? Ensure Optimal High-Temp Performance
- What is the function of a Tube Furnace in the thermal oxidation of Ti6Al4V alloy? Enhance Hardness & Wear Resistance
- What is the function of quartz tube vacuum sealing in Fe3GaTe2 crystal growth? Achieve High-Purity Results
- What is the role of a laboratory tube furnace in the heat treatment of Zr-2.5%Nb pressure tube samples? (550°C-800°C)
- What materials are commonly used for furnace tube construction and why? Choose the Right Tube for Your Lab's Needs



















