The gas path control system acts as the critical regulator of the reaction environment. It functions by delivering a continuous, precise flow of inert gas, typically nitrogen, to purge oxygen from the furnace chamber and sweep away volatile byproducts generated during pyrolysis.
The gas path control system is the difference between creating high-performance activated carbon and simply burning material into ash; it maintains the strict inert atmosphere required to etch micropores into the carbon skeleton without triggering oxidative combustion.

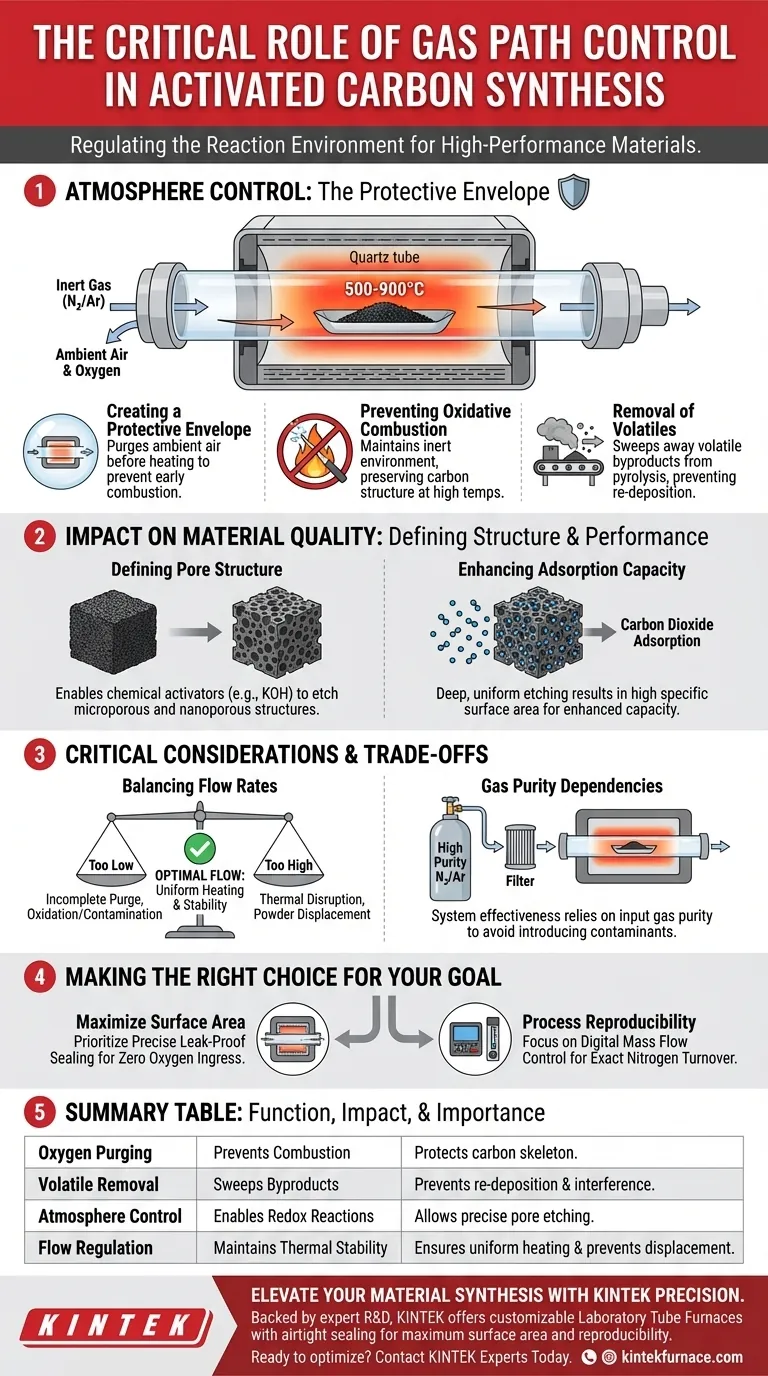
The Role of Atmosphere Control
Creating a Protective Envelope
The primary function of the gas path control system is to establish and maintain a protective atmosphere.
Before heating begins, the system must effectively purge ambient air from the tube.
If this step fails, oxygen remaining in the chamber will react with the carbon substrate as temperatures rise, leading to combustion rather than activation.
Preventing Oxidative Combustion
Synthesis occurs at high temperatures, typically between 500 and 900 degrees Celsius.
In an uncontrolled atmosphere, these temperatures would cause the carbon material to burn away completely.
By delivering a constant flow of nitrogen or argon, the system ensures the environment remains inert, preserving the carbon structure for processing.
Removal of Volatiles
During pyrolysis, the raw material breaks down and releases volatile components.
The gas path control system acts as a transport mechanism, continuously sweeping these gases out of the hot zone.
This prevents volatile byproducts from re-depositing on the material or interfering with the delicate chemical activation process.
Impact on Material Quality
Defining Pore Structure
The ultimate goal of activated carbon synthesis is to achieve a high specific surface area.
The gas control system enables chemical activators, such as potassium hydroxide (KOH), to react with the carbon substrate through redox reactions.
This precise interaction etches the carbon skeleton, creating the abundant microporous and nanoporous structures that define high-quality activated carbon.
Enhancing Adsorption Capacity
The porosity generated by this controlled environment directly dictates the material's performance.
A stable atmosphere allows for deep, uniform etching.
This results in a final product with significantly enhanced capacity for tasks like carbon dioxide adsorption.
Critical Considerations and Trade-offs
Balancing Flow Rates
While flow is essential, "more" is not always better.
A flow rate that is too low may fail to fully evacuate oxygen or volatile gases, leading to material contamination or oxidation.
Conversely, an excessively high flow rate can disrupt the thermal stability of the specific temperature zone (e.g., cooling the sample surface) or physically disturb fine powders.
Gas Purity Dependencies
The control system is only as effective as the gas source it regulates.
Even a perfectly calibrated control system will fail if the input gas contains impurities or moisture.
Users must ensure the nitrogen or argon source is of high purity to prevent introducing contaminants that the control system cannot filter out.
Making the Right Choice for Your Goal
To maximize the effectiveness of your laboratory tube furnace during synthesis, align your gas control strategy with your specific objectives:
- If your primary focus is maximizing surface area: Prioritize a system with precise leak-proof sealing to ensure zero oxygen ingress during the crucial KOH etching phase.
- If your primary focus is process reproducibility: Focus on a control system that offers digital mass flow control to guarantee the exact same nitrogen turnover rate for every batch.
The gas path control system is not merely an accessory; it is the physical foundation that allows high-surface-area carbon to exist.
Summary Table:
| Function | Impact on Synthesis | Why It Matters |
|---|---|---|
| Oxygen Purging | Prevents combustion | Protects the carbon skeleton from burning to ash at high temperatures. |
| Volatile Removal | Sweeps pyrolysis byproducts | Prevents re-deposition of volatiles and interference with chemical activation. |
| Atmosphere Control | Enables redox reactions | Allows chemical activators (like KOH) to etch the precise microporous structure. |
| Flow Regulation | Maintains thermal stability | Ensures uniform heating and prevents fine powder displacement during processing. |
Elevate Your Material Synthesis with KINTEK Precision
Don't let poor atmosphere control compromise your research. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed specifically for high-precision applications like activated carbon synthesis. Backed by expert R&D and manufacturing, our laboratory furnaces offer customizable gas path control and airtight sealing to ensure your materials achieve maximum surface area and reproducibility.
Ready to optimize your lab's high-temperature processes?
Visual Guide
References
- Lai Thi Hoan, Duong Duc La. Sustainable Removal of Phenol from Aqueous Media by Activated Carbon Valorized from Polyethyleneterephthalate (PET) Plastic Waste. DOI: 10.3390/su17020548
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a laboratory high-temperature tube resistance furnace contribute to the aging treatment of Ni-W-Co-Ta alloys?
- What synthesis environment does a vacuum tube furnace provide for C@TiC nanoparticles? Master Oxygen-Free Pyrolysis
- What functions do high vacuum pumping systems and tube furnaces serve? Enhancing Amorphous Ribbon Performance
- What is the function of a high-temperature tube furnace in Cu(111) transformation? Achieve Atomic Precision
- What are the key features of a modern tube furnace? Precision, Control, and Versatility for Advanced Labs
- How does annealing in a laboratory tube furnace affect In2Se3 quality? Achieve Phase Stabilization & Purity
- What are the key benefits of using a tube furnace for material processing? Achieve Precise Heat Control for Superior Results
- What specific conditions does a tube furnace provide for the low-temperature exsolution of cobalt? Optimize Performance



















