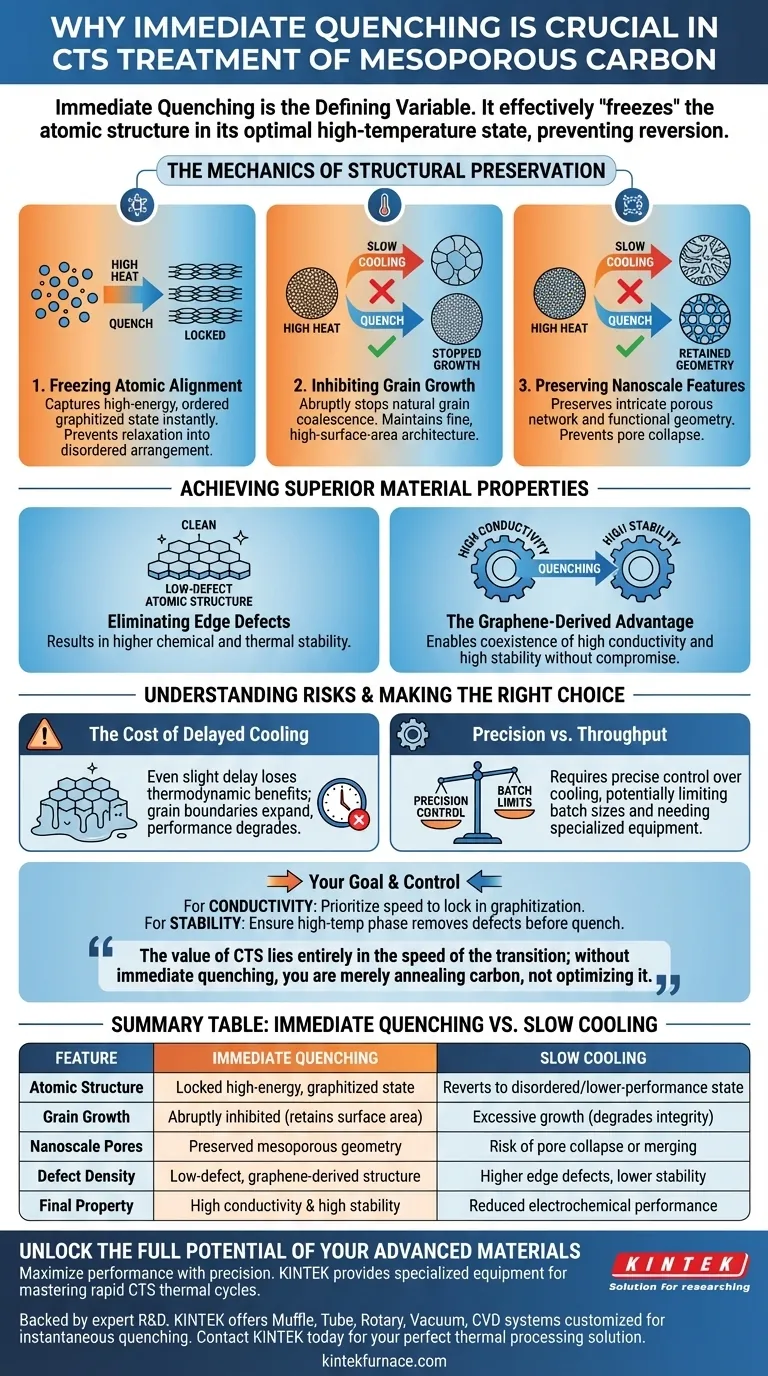
Immediate quenching is the defining variable that dictates the final quality of Carbon Thermal Shock (CTS) treated material. By rapidly dropping the temperature, you effectively "freeze" the atomic structure in its optimal high-temperature state, preventing the material from reverting to a less desirable configuration.
The core purpose of immediate quenching is to lock in a highly graphitized, low-defect structure before natural cooling can alter it. This rapid thermal cycle is the only way to inhibit excessive grain growth while simultaneously preserving the material's critical nanoscale mesoporous features.

The Mechanics of Structural Preservation
Freezing Atomic Alignment
During the high-temperature phase of CTS, the carbon atoms arrange themselves into a highly graphitized structure. This alignment is responsible for superior electrical conductivity.
Immediate quenching acts as a sudden brake on atomic movement. It captures this high-energy, ordered state instantly, ensuring the carbon does not relax into a disordered or lower-performance arrangement.
Inhibiting Grain Growth
Heat naturally encourages grains within the material to coalesce and grow larger. If the material were allowed to cool slowly, excessive grain growth would occur.
Large grains inevitably degrade the material's surface area and structural integrity. Quenching stops this growth abruptly, maintaining the fine, high-surface-area architecture required for high-performance applications.
Preserving Nanoscale Features
The utility of this carbon relies heavily on its mesoporous characteristics. These are tiny nanoscale pores that provide vast surface area.
Slow cooling processes threaten to collapse or merge these pores. Rapid quenching preserves the intricate porous network, ensuring the material retains its functional geometry.
Achieving Superior Material Properties
Eliminating Edge Defects
Standard processing often leaves carbon materials with structural imperfections known as edge defects. The CTS process, tailored with immediate quenching, creates a low-defect atomic structure.
The result is a "cleaner" material on the atomic level. This directly translates to higher chemical and thermal stability in the final product.
The Graphene-Derived Advantage
When executed correctly, this process produces graphene-derived mesoporous carbon. This specific classification of carbon is highly sought after because it bridges two usually conflicting properties.
It offers high conductivity (due to graphitization) alongside high stability (due to the lack of defects). Only the thermal shock of immediate quenching allows these two properties to coexist without compromise.
Understanding the Process Risks
The Cost of Delayed Cooling
The primary pitfall in this process is a lack of speed. If the quenching is not instantaneous, the thermodynamic benefits are lost.
Even a slight delay allows grain boundaries to expand. This results in a material that may look similar macroscopically but lacks the conductive and structural performance of true CTS-treated carbon.
Precision vs. Throughput
Achieving this ultra-fast thermal cycling requires precise control over the cooling medium and timing.
This adds complexity to the manufacturing process. The strict requirement for immediate temperature drops can limit batch sizes or require specialized equipment compared to standard slow-cooling annealing methods.
Making the Right Choice for Your Goal
To maximize the benefits of CTS-treated carbon, you must align your processing controls with your specific performance targets.
- If your primary focus is Electrical Conductivity: Prioritize the speed of the quench to lock in the maximum degree of graphitization without allowing relaxation.
- If your primary focus is Long-Term Stability: Ensure the high-temperature phase is sufficient to remove edge defects before the quench freezes the structure.
The value of CTS lies entirely in the speed of the transition; without immediate quenching, you are merely annealing carbon, not optimizing it.
Summary Table:
| Feature | Immediate Quenching Effect | Slow Cooling Result |
|---|---|---|
| Atomic Structure | Locked in high-energy, graphitized state | Reverts to disordered/lower-performance state |
| Grain Growth | Abruptly inhibited (retains surface area) | Excessive growth (degrades integrity) |
| Nanoscale Pores | Preserved mesoporous geometry | Risk of pore collapse or merging |
| Defect Density | Low-defect, graphene-derived structure | Higher edge defects and lower stability |
| Final Property | High conductivity & high stability | Reduced electrochemical performance |
Unlock the Full Potential of Your Advanced Materials
Maximize the performance of your graphene-derived carbons with the precision they demand. KINTEK provides the specialized equipment needed to master the rapid thermal cycles of CTS treatment.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable to ensure the instantaneous quenching and precise temperature control required for your unique research goals.
Don't let slow cooling compromise your results. Contact KINTEK today to find the perfect thermal processing solution for your laboratory.
Visual Guide
References
- Mitesh Ganpat Mapari, Tae Young Kim. Edge‐Free Graphene‐Derived Mesoporous Carbon for High‐Voltage Supercapacitors. DOI: 10.1002/sstr.202500265
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why must high-purity nitrogen be used for biochar activation? Ensure Carbon Integrity and Pore Development
- What is the main benefit of using a benchtop industrial oven? Save Space and Boost Efficiency in Your Lab
- What is the purpose of using controlled anaerobic environments for peat carbonization? Unlock High-Energy Industrial Fuel
- Why is specialized dewaxing and annealing necessary for glass-to-metal seals? Ensure Hermeticity and Clarity
- What is the function of ball milling in Li-NASICON synthesis? Optimize Your Solid Electrolyte Performance
- How do stirring equipment and temperature-controlled heating stages influence magnetic nanoparticle quality?
- What role does thermal annealing play in the post-treatment of CZTSSe nanocrystals? Optimize Phase Purity & Crystallinity
- Why is it necessary to use a vacuum drying oven for porous graphene cathodes? Ensure Peak Battery Performance



















