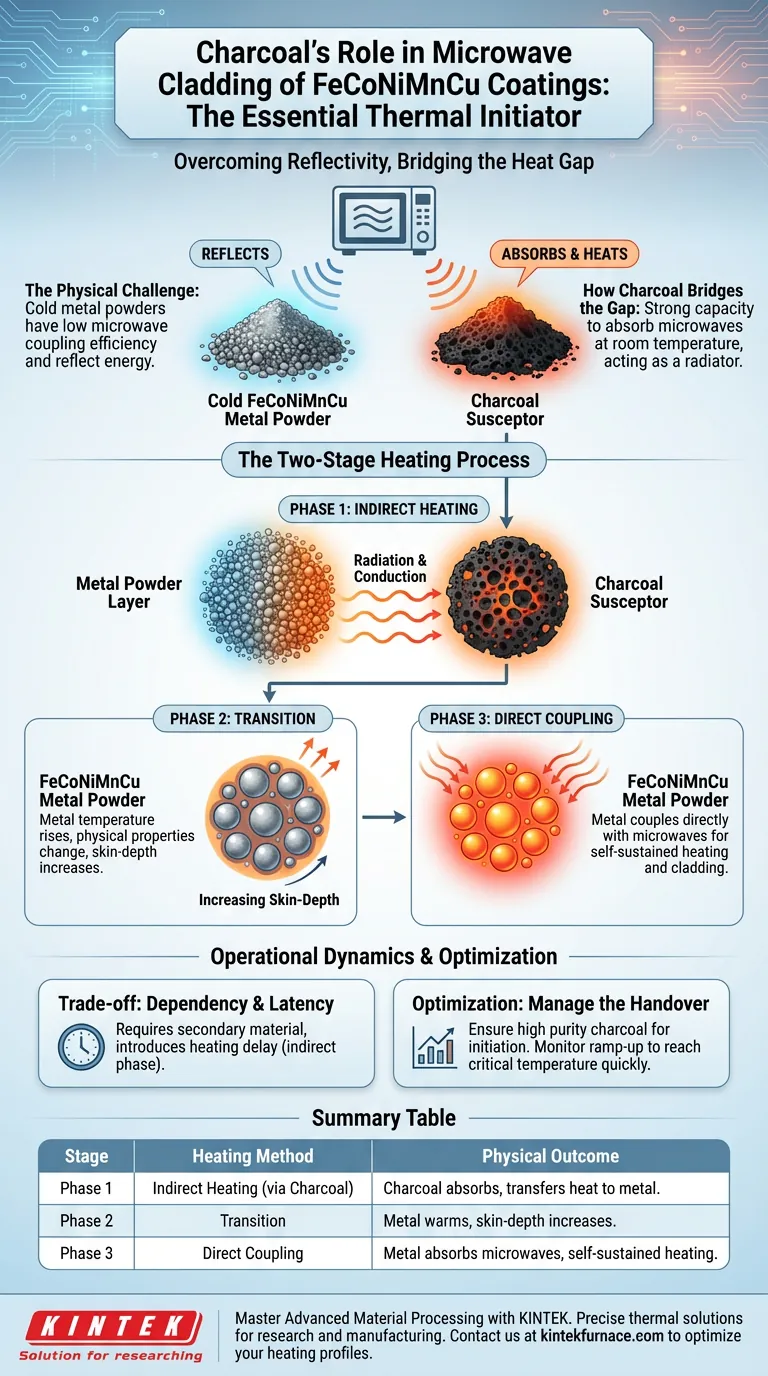
Charcoal functions as an essential thermal initiator. It is utilized because metal powders, such as FeCoNiMnCu, naturally reflect microwaves at room temperature, preventing them from heating up effectively on their own. Charcoal solves this by absorbing microwave energy immediately, converting it to heat, and transferring that thermal energy to the metal powder until the metal reaches a state where it can absorb the microwaves directly.
Microwave cladding of metals relies on a distinct two-stage heating process. Charcoal acts as the "starter motor," overcoming the low coupling efficiency of cold metal powders to trigger their ability to generate their own heat.
The Physical Challenge of Metal Powders
The Problem of Reflectivity
At room temperature, metal powders possess a low microwave coupling efficiency.
Instead of absorbing the energy, the FeCoNiMnCu powder reflects the microwaves. Without an external intervention, the material would remain too cool to process.
How Charcoal Bridges the Gap
High Absorption Capacity
Charcoal is selected as a susceptor because it behaves differently than metal.
It possesses a strong capacity to absorb microwaves at room temperature. Upon exposure, it immediately begins to convert microwave energy into thermal energy.
Heat Transfer Mechanisms
Once the charcoal heats up, it acts as a localized radiator.
It transfers its generated heat to the adjacent metal powder layer. This transfer occurs through a combination of radiation and conduction, steadily raising the temperature of the metal powder.
Reaching the Critical State
Increasing Skin-Depth
The ultimate goal of the charcoal is to raise the metal powder to a critical temperature.
As the temperature rises, the physical properties of the metal powder change. Specifically, the skin-depth of the metal increases.
Transition to Direct Coupling
Once this skin-depth increases sufficiently, the dynamic shifts.
The metal powder is no longer purely reflective; it begins to couple directly with the microwaves. At this stage, the metal generates its own heat, completing the cladding process that the charcoal initiated.
Operational Dynamics and Trade-offs
Dependency on Auxiliary Materials
The primary trade-off in this process is the requirement for a secondary material.
The process is not self-starting; it is entirely dependent on the susceptor’s efficiency. If the charcoal fails to absorb energy or transfer heat effectively, the metal will never reach the state required for direct coupling.
The Two-Step Efficiency Gap
This method introduces a latency period in the heating profile.
Energy is first spent heating the charcoal before it heats the metal. This indirect heating phase is necessary but represents a delay compared to materials that can couple directly at room temperature.
Optimizing the Cladding Process
To ensure successful microwave cladding of FeCoNiMnCu, you must manage the transition between indirect and direct heating.
- If your primary focus is process initiation: Ensure your susceptor material (charcoal) has high purity to maximize immediate microwave absorption at room temperature.
- If your primary focus is process efficiency: Monitor the ramp-up time closely; the goal is to reach the metal's critical temperature as quickly as possible to switch to direct heating.
Understanding this thermal handover is the key to mastering microwave processing of reflective metals.
Summary Table:
| Stage | Heating Method | Material Role | Physical Outcome |
|---|---|---|---|
| Phase 1 | Indirect Heating | Charcoal absorbs microwave energy | Temperature rises via radiation/conduction |
| Phase 2 | Transition | Metal powder warms up | Skin-depth of metal increases |
| Phase 3 | Direct Coupling | FeCoNiMnCu absorbs microwaves | Self-sustained heating for cladding process |
Master Advanced Material Processing with KINTEK
Precise thermal management is the foundation of high-performance coatings. At KINTEK, we empower researchers and manufacturers with state-of-the-art thermal solutions. Whether you are performing microwave cladding or complex high-temp synthesis, our expert R&D and manufacturing teams provide:
- Customizable Muffle, Tube, and Vacuum Furnaces designed for unique material needs.
- Advanced CVD and Rotary Systems for uniform layer deposition.
- Expert technical support to optimize your heating profiles and process efficiency.
Ready to elevate your lab's capabilities? Contact us today to discuss your project requirements!
Visual Guide
References
- Shubham Sharma, Emad A. A. Ismail. Investigation of surface hardness, thermostability, tribo-corrosion, and microstructural morphological properties of microwave-synthesized high entropy alloy FeCoNiMnCu coating claddings on steel. DOI: 10.1038/s41598-024-55331-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano Diamond Coating
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is immediate water-quenching required after thermal simulation? Preserve (CoCrNi)94Al3Ti3 Alloy Microstructure
- What role does a high-temperature annealing furnace play in the preparation of AAO substrates? Enhance Pore Regularity
- What is the significance of the 220 °C annealing process? Unlock High-Purity Anti-Perovskite Thin Film Synthesis
- What is the significance of using different sizes of steel working ampoules? Precision vs. Efficiency in Lab Research
- How does a vacuum drying oven contribute to the study of the hydration degree in cement pastes? Essential Lab Insights
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- Why is a 1:1 mixture of NaNO3 and KNO3 used in molten salt baths? Optimize Quenching Performance
- What is the function of a drying oven in the chemical activation of biochar with phosphoric acid? Optimize Biochar Quality



















