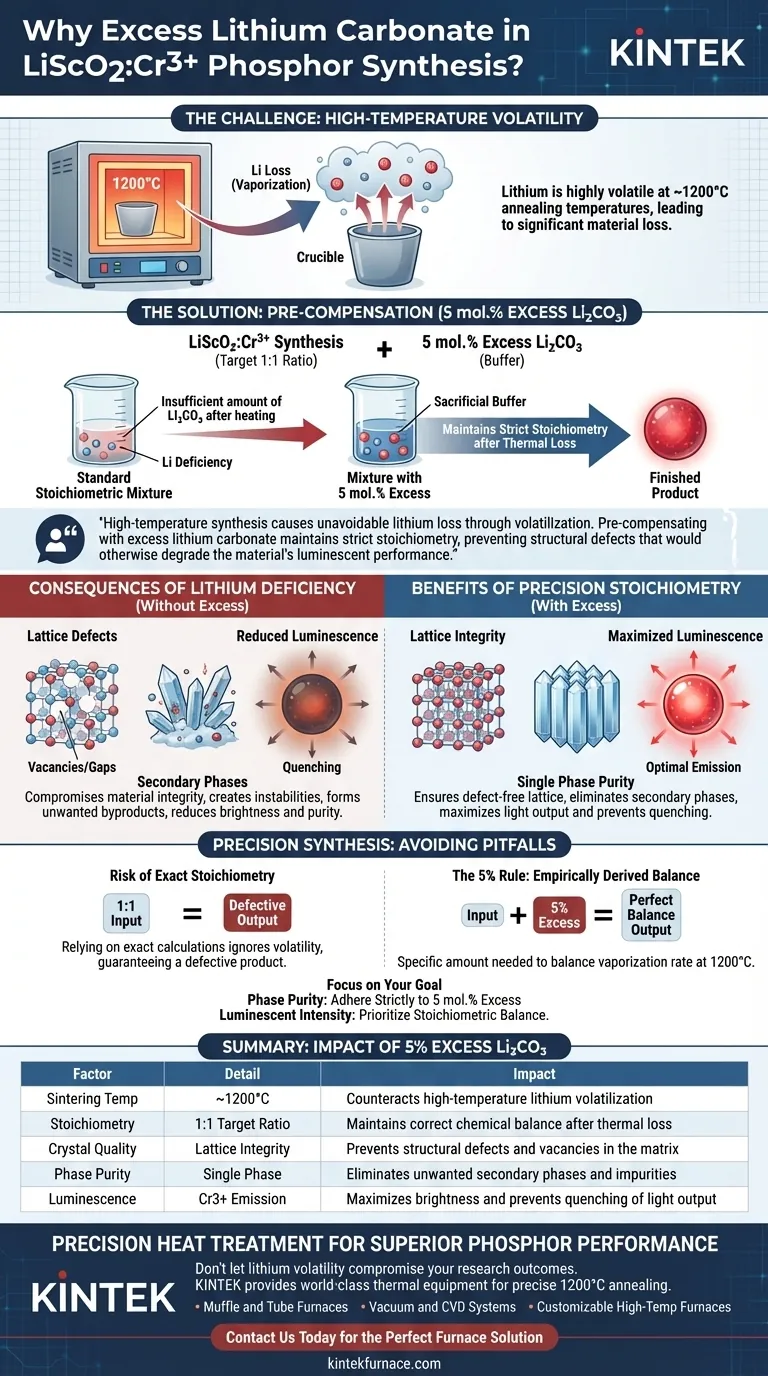
The primary reason for adding excess lithium carbonate is to compensate for the high volatility of lithium when subjected to extreme heat. During the synthesis of $LiScO_2:Cr^{3+}$, the materials undergo high-temperature annealing at approximately 1200°C, causing a significant portion of the lithium to vaporize. To counteract this inevitable loss, researchers introduce an excess amount—typically 5 mol.%—to ensure the final chemical composition remains balanced.
High-temperature synthesis causes unavoidable lithium loss through volatilization. Pre-compensating with excess lithium carbonate maintains strict stoichiometry, preventing structural defects that would otherwise degrade the material's luminescent performance.

The Challenge of High-Temperature Synthesis
Creating high-quality phosphors requires precise chemical reactions, but the physical properties of lithium introduce specific challenges during heating.
Lithium Volatility at 1200°C
Lithium is a volatile element, meaning it readily transitions into a vapor phase at high temperatures.
In the preparation of $LiScO_2:Cr^{3+}$, the annealing process often reaches 1200°C. At this thermal extreme, a standard stoichiometric mixture (an exact 1:1 ratio) would result in a final product with a lithium deficiency because a portion of the element simply evaporates.
The Role of Pre-Compensation
To address this, researchers deliberately "overload" the initial mixture with lithium carbonate.
By adding approximately 5 mol.% excess, they provide a buffer. This extra material is calculated to sacrifice itself to volatilization, leaving behind the exact amount required to form the correct crystal lattice.
Consequences of Lithium Deficiency
Failing to add excess lithium does not just result in a smaller yield; it fundamentally alters the quality of the material.
Preventing Lattice Defects
If the lithium content drops below the required amount, the crystal structure (lattice) of the material will contain vacancies or gaps.
These structural imperfections are known as lattice defects. They compromise the integrity of the host material, creating instability within the crystal matrix.
Avoiding Secondary Phases
When the ratio of ingredients is incorrect, the chemical reaction may produce unwanted byproducts.
A lack of lithium can lead to the formation of secondary phases—different crystalline compounds that are not $LiScO_2$. These impurities contaminate the sample and disrupt the uniformity of the phosphor.
Safeguarding Luminescence Purity
The ultimate goal of this synthesis is to create a material that emits light (luminescence) effectively.
The $Cr^{3+}$ ions responsible for this emission require a precise structural environment to function. Lattice defects and secondary phases act as "quenchers" or disturbances, significantly reducing the brightness and purity of the luminescence.
Common Pitfalls in Synthesis
While adding excess material is a solution, it requires precision to avoid introducing new problems.
The Risk of Exact Stoichiometry
A common mistake in solid-state synthesis is assuming that "input equals output."
In this specific reaction, relying on exact stoichiometric calculations without accounting for volatility is a critical error. It guarantees a defective product with poor optical performance.
The Specificity of the 5% Rule
The 5 mol.% figure is not arbitrary; it is an empirically derived value.
It represents the specific amount needed to balance the vaporization rate at 1200°C. Deviating significantly from this percentage—either by adding too little or potentially too much—could result in either defects (from deficiency) or unreacted flux (from excessive surplus).
Making the Right Choice for Your Goal
When synthesizing volatile compounds like $LiScO_2:Cr^{3+}$, understanding the thermal behavior of your reactants is as important as the formula itself.
- If your primary focus is Phase Purity: Adhere strictly to the 5 mol.% excess guideline to prevent the formation of secondary impurity phases caused by lithium vacancies.
- If your primary focus is Luminescent Intensity: Prioritize the stoichiometric balance to ensure the Chromium activators sit in a defect-free lattice, maximizing light output.
By anticipating material loss before it happens, you ensure the integrity and performance of your final phosphor.
Summary Table:
| Factor | Detail | Impact of 5% Excess Li2CO3 |
|---|---|---|
| Sintering Temp | ~1200°C | Counteracts high-temperature lithium volatilization |
| Stoichiometry | 1:1 Target Ratio | Maintains the correct chemical balance after thermal loss |
| Crystal Quality | Lattice Integrity | Prevents structural defects and vacancies in the matrix |
| Phase Purity | Single Phase | Eliminates unwanted secondary phases and impurities |
| Luminescence | Cr3+ Emission | Maximizes brightness and prevents quenching of light output |
Precision Heat Treatment for Superior Phosphor Performance
Don't let lithium volatility compromise your research outcomes. At KINTEK, we understand that high-quality $LiScO_2:Cr^{3+}$ synthesis demands both precise chemistry and world-class thermal equipment.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab solutions, including:
- Muffle and Tube Furnaces for precise 1200°C annealing.
- Vacuum and CVD Systems for advanced material synthesis.
- Customizable High-Temp Furnaces tailored to your unique stoichiometric requirements.
Whether you are focusing on phase purity or luminescent intensity, our equipment provides the thermal stability needed to prevent structural defects. Contact us today to find the perfect furnace for your laboratory!
Visual Guide
References
- Leoni Frehmeyer, Thomas Jüstel. On the optimisation of the broadband NIR emitter LiScO2:Cr3+. DOI: 10.6001/chemija.2025.36.2.5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Stainless Steel KF ISO Vacuum Flange Blind Plate for High Vacuum Systems
People Also Ask
- What are the advantages of using the DO radiation model in high-temp furnaces? Boost Precision & Emission Control
- What advantages does a microwave sintering furnace offer for LLZTO? Speed and Performance Compared
- How are electric furnaces applied in powder metallurgy and 3D printing? Unlock Precision Sintering and Heat Treatment
- What is the primary function of a vacuum drying oven? Key to Composite Anode Slurry Preparation
- Why is a specific glass slide used to cover Zinc powder? Mastering ZnO Nanostructure Precision
- How does a heated substrate platform mitigate the coffee ring effect? Enhance Ag2Se Printing Precision
- What is the function of a high-temperature heating reactor in OPF delignification? Unlock High-Purity Cellulose
- How do thermal stripping tools and heating equipment facilitate solar panel recycling? High-Value Glass Recovery Guide



















