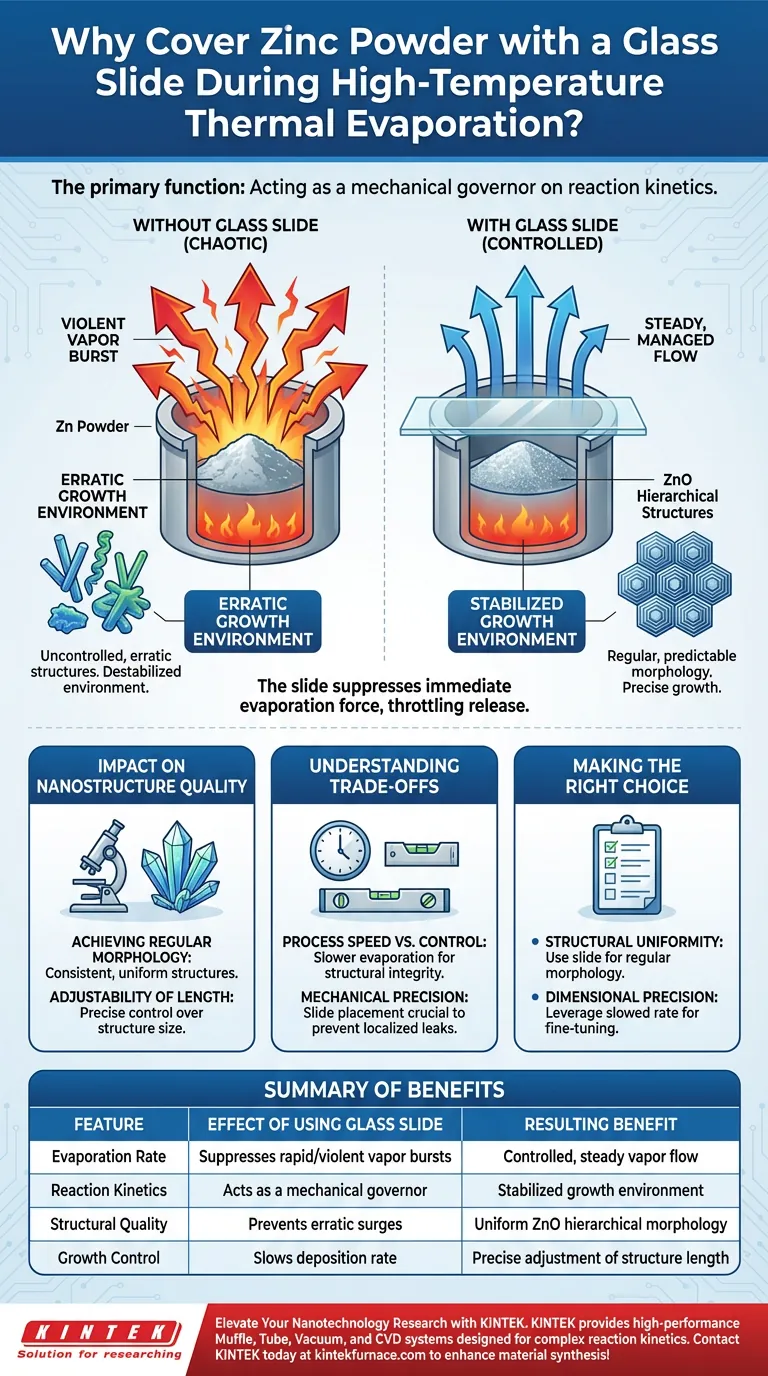
The primary function of the glass slide is to act as a mechanical governor on the reaction kinetics of Zinc powder. During high-temperature thermal evaporation, Zinc is prone to rapid and violent phase changes; placing a blank glass slide over the powder physically suppresses this volatility. This creates a regulated environment where the release rate of Zinc vapor is slowed and stabilized.
By converting a chaotic vapor burst into a controlled release, the glass slide stabilizes the reaction environment, enabling the precise growth of ZnO hierarchical structures with consistent morphology.
Managing Reaction Kinetics
The Problem of Violent Evaporation
At high temperatures, Zinc powder does not simply evaporate; it undergoes a rapid and often violent transition. Without containment, this results in an erratic surge of vapor that destabilizes the entire growth environment.
The Slide as a Physical Barrier
The blank glass slide is placed directly over the source to counter this volatility. It acts as a lid, suppressing the immediate force of the evaporation.
This does not stop the reaction, but rather throttles the release of Zinc vapor. It transforms an unpredictable explosion of material into a steady, manageable flow.
Impact on Nanostructure Quality
Achieving Regular Morphology
Structure quality is directly downstream of reaction stability. Because the glass slide regulates the vapor release, the growth environment remains constant.
This stability allows for the formation of ZnO hierarchical structures that possess regular, predictable morphologies. Without this regulation, the structures would likely be deformed or inconsistent.
Adjustability of Length
The "brakes" applied by the glass slide give the operator greater control over the outcome.
By slowing the rate of vapor release, the system allows for the adjustment of structure lengths. This precise control turns a chemical reaction into an engineering process.
Understanding the Trade-offs
Process Speed vs. Control
The use of a physical cover inherently creates a bottleneck in vapor flow. While this is necessary for quality, it means the evaporation process will be slower than an uncovered reaction. You are trading rapid deposition for structural integrity.
Mechanical Precision
The effectiveness of this method relies on the physical placement of the slide. If the slide acts as a barrier, it must be positioned correctly to prevent localized "leaks" of high-pressure vapor, which would reintroduce instability to the system.
Making the Right Choice for Your Goal
To determine if this setup is required for your specific application, consider your end goals:
- If your primary focus is Structural Uniformity: Use the glass slide to suppress violent evaporation and ensure the ZnO structures maintain a regular morphology.
- If your primary focus is Dimensional Precision: Leverage the slowed reaction rate to fine-tune and adjust the specific lengths of the hierarchical structures.
Mastering the mechanical suppression of vapor release is the key to transitioning from chaotic chemical reactions to precision nanostructure engineering.
Summary Table:
| Feature | Effect of Using Glass Slide | Resulting Benefit |
|---|---|---|
| Evaporation Rate | Suppresses rapid/violent vapor bursts | Controlled, steady vapor flow |
| Reaction Kinetics | Acts as a mechanical governor | Stabilized growth environment |
| Structural Quality | Prevents erratic surges | Uniform ZnO hierarchical morphology |
| Growth Control | Slows deposition rate | Precise adjustment of structure length |
Elevate Your Nanotechnology Research with KINTEK
Precision in thermal evaporation starts with the right equipment. KINTEK provides high-performance Muffle, Tube, Vacuum, and CVD systems designed to support complex reaction kinetics like ZnO nanostructure growth. Backed by expert R&D and manufacturing, our systems are fully customizable to meet the unique mechanical and thermal stability requirements of your laboratory.
Ready to transform chaotic chemical reactions into precision engineering? Contact KINTEK today to discover how our high-temperature furnaces can enhance your material synthesis!
Visual Guide
References
- Mingjin Liu, Yu‐Lun Chueh. Rational design of comb-like 1D–1D ZnO–ZnSe heterostructures toward their excellent performance in flexible photodetectors. DOI: 10.1039/d3nr06617g
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the drawbacks of large industrial ovens? Avoid Costly Inefficiencies and Boost Productivity
- What is the use of high temperature furnace? Transform Materials with Precision Thermal Processing
- What is the function of a heated tundish in a metal powder production system? Optimize Flow and Thermal Consistency
- Why is preheating a metal mold to 660 °C necessary for Al/Cu bimetallic composites? Unlock Strong Chemical Bonding
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- What is the primary function of an industrial drying oven for GBC? Achieving Material Standardization and Quality
- How does a multi speed furnace work? Achieve Ultimate Comfort & Efficiency
- What hardware characteristics are required for a reactor system to support a three-step redox process in chemical looping?









