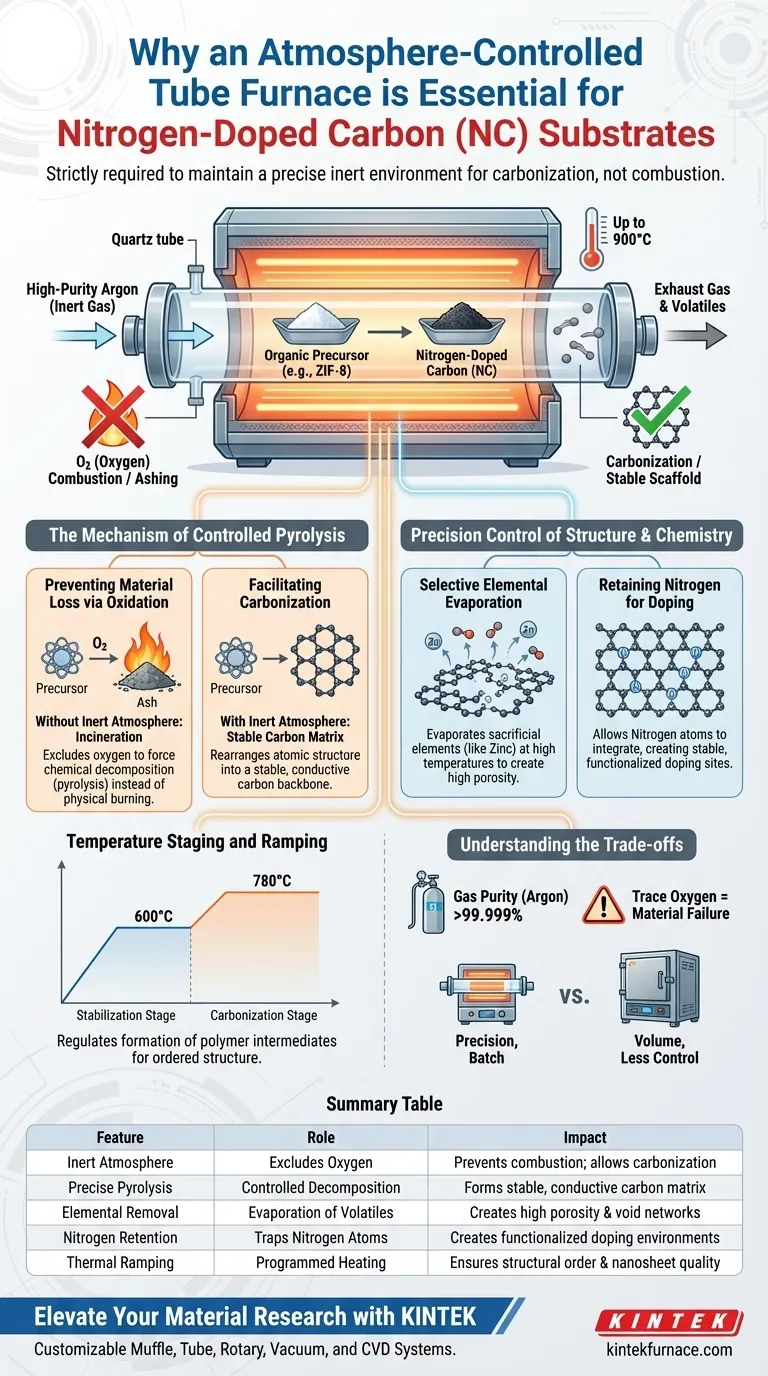
An atmosphere-controlled tube furnace is strictly required because it maintains a precise inert environment, typically high-purity argon, which allows organic precursors to undergo carbonization rather than oxidative combustion. Without this oxygen-free protection during high-temperature treatment (often reaching 900°C), the precursor materials would simply burn away, preventing the formation of the stable, porous carbon scaffold necessary for nitrogen-doped substrates.
The tube furnace functions not just as a heater, but as a selective chemical reactor. It creates the specific thermodynamic conditions needed to evaporate sacrificial elements (like Zinc) while effectively trapping nitrogen atoms within the carbon lattice to create a functionalized, high-porosity material.

The Mechanism of Controlled Pyrolysis
Preventing Material Loss via Oxidation
The fundamental challenge in creating carbon substrates is that organic precursors are highly flammable at elevated temperatures.
If exposed to oxygen at 900°C, the framework would incinerate, leaving behind only ash. The tube furnace excludes oxygen entirely, forcing the material to decompose chemically (pyrolysis) rather than physically burn.
Facilitating Carbonization
Once the atmosphere is secured, the furnace drives the transformation of the organic framework into a rigid carbon structure.
This process, known as carbonization, rearranges the atomic structure. It converts the precursor into a stable, conductive carbon matrix that serves as the physical backbone for the substrate.
Precision Control of Structure and Chemistry
Selective Elemental Evaporation
For precursors like ZIF-8, the furnace facilitates a critical separation process.
At high temperatures, volatile metallic elements such as Zinc are evaporated out of the material. This controlled removal is essential because it leaves behind a network of voids, directly creating the material's high porosity.
Retaining Nitrogen for Doping
While Zinc is expelled, the furnace environment allows Nitrogen atoms to remain.
These nitrogen atoms do not evaporate; instead, they integrate into the carbon scaffold. This creates a stable nitrogen coordination environment, which is the defining characteristic of a "nitrogen-doped" substrate.
Temperature Staging and Ramping
The tube furnace allows for programmed temperature profiles, which is vital for complex precursors.
For example, a two-stage heating strategy (e.g., stabilizing at 600°C before rising to 780°C) allows for the orderly formation of polymer intermediates. This regulation ensures the final nanosheets have the correct chemical structure rather than a chaotic, amorphous arrangement.
Understanding the Trade-offs
Sensitivity to Gas Purity
The success of this process is entirely dependent on the quality of the inert atmosphere.
Even trace amounts of oxygen due to a leak or low-quality argon can compromise the "hard carbon" structure or lead to partial surface oxidation. The system requires rigorous sealing and high-purity gas sources.
Throughput vs. Precision
Tube furnaces are inherently limited in volume compared to industrial box furnaces.
While they offer exceptional control over atmosphere and heating rates—essential for doping sulfur or nitrogen—they are typically batch-process tools. Scaling this process up for mass production requires significant engineering to maintain the same atmospheric uniformity.
Making the Right Choice for Your Goal
To maximize the quality of your nitrogen-doped carbon (NC) substrates, align your furnace parameters with your specific structural targets:
- If your primary focus is High Porosity: Ensure your maximum temperature reaches the evaporation point of your sacrificial element (e.g., 900°C for Zinc removal) to maximize void creation.
- If your primary focus is Specific Surface Area: Consider introducing activating agents like Carbon Dioxide (CO2) at high temperatures (1000°C) to physically etch structural defects into the matrix.
- If your primary focus is Chemical Stability: Utilize a programmed, multi-stage heating ramp to allow carbon polymer intermediates to form bonds slowly before the final high-temperature carbonization.
The tube furnace is the gatekeeper that determines whether your precursor becomes a pile of ash or a highly engineered catalytic substrate.
Summary Table:
| Feature | Role in NC Substrate Preparation | Impact on Material Properties |
|---|---|---|
| Inert Atmosphere | Excludes oxygen using high-purity Argon | Prevents combustion; allows carbonization |
| Precise Pyrolysis | Controlled chemical decomposition | Forms a stable, conductive carbon matrix |
| Elemental Removal | Facilitates evaporation of Zinc/volatile metals | Creates high porosity and void networks |
| Nitrogen Retention | Traps Nitrogen atoms in the carbon lattice | Creates functionalized doping environments |
| Thermal Ramping | Programmed multi-stage heating profiles | Ensures structural order and nanosheet quality |
Elevate Your Material Research with KINTEK
Precision is the difference between a successful nitrogen-doped substrate and material loss. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your unique research needs.
Our systems provide the rigorous atmospheric sealing and precise temperature staging required to achieve high-porosity, high-quality NC substrates. Contact us today to discover how KINTEK’s advanced furnace technology can optimize your carbonization and doping processes!
Visual Guide
References
- Junjun Pei, Jinming Luo. Non-metallic iodine single-atom catalysts with optimized electronic structures for efficient Fenton-like reactions. DOI: 10.1038/s41467-025-56246-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the maximum temperature for a tube furnace? Unlock the Right Heat for Your Application
- What are the benefits of continuous movement of the sample in a rotary tube furnace? Achieve Superior Uniformity and Efficiency
- What core functions does a tube high-temperature furnace perform? Mastering In-situ Carbothermal Reduction
- What are the differences between solid and split tube furnaces? Choose the Right Furnace for Your Lab
- What are the key features of a split tube furnace? Unlock Superior Access and Control for Complex Samples
- What critical conditions does a high-precision tube furnace provide? Optimize Catalyst Reduction & Particle Control
- Why is the control of heating and cooling rates in a tube furnace critical for the thermal reduction of lithium niobate?
- What are the benefits of using a high vacuum tube furnace for Ti-Si-C-Mo coatings? Maximize Coating Performance



















