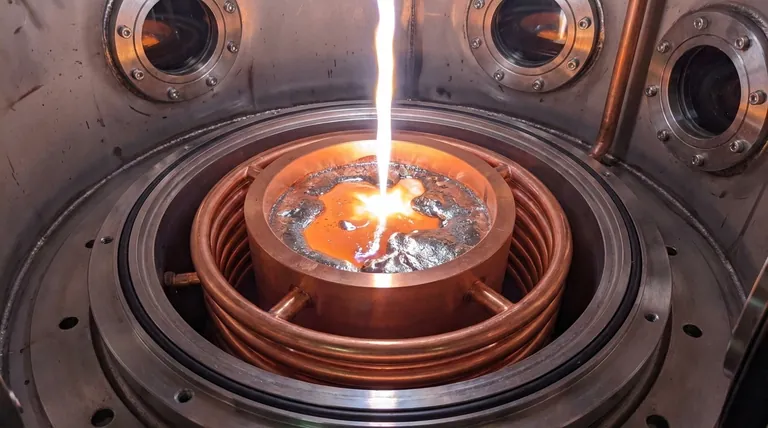
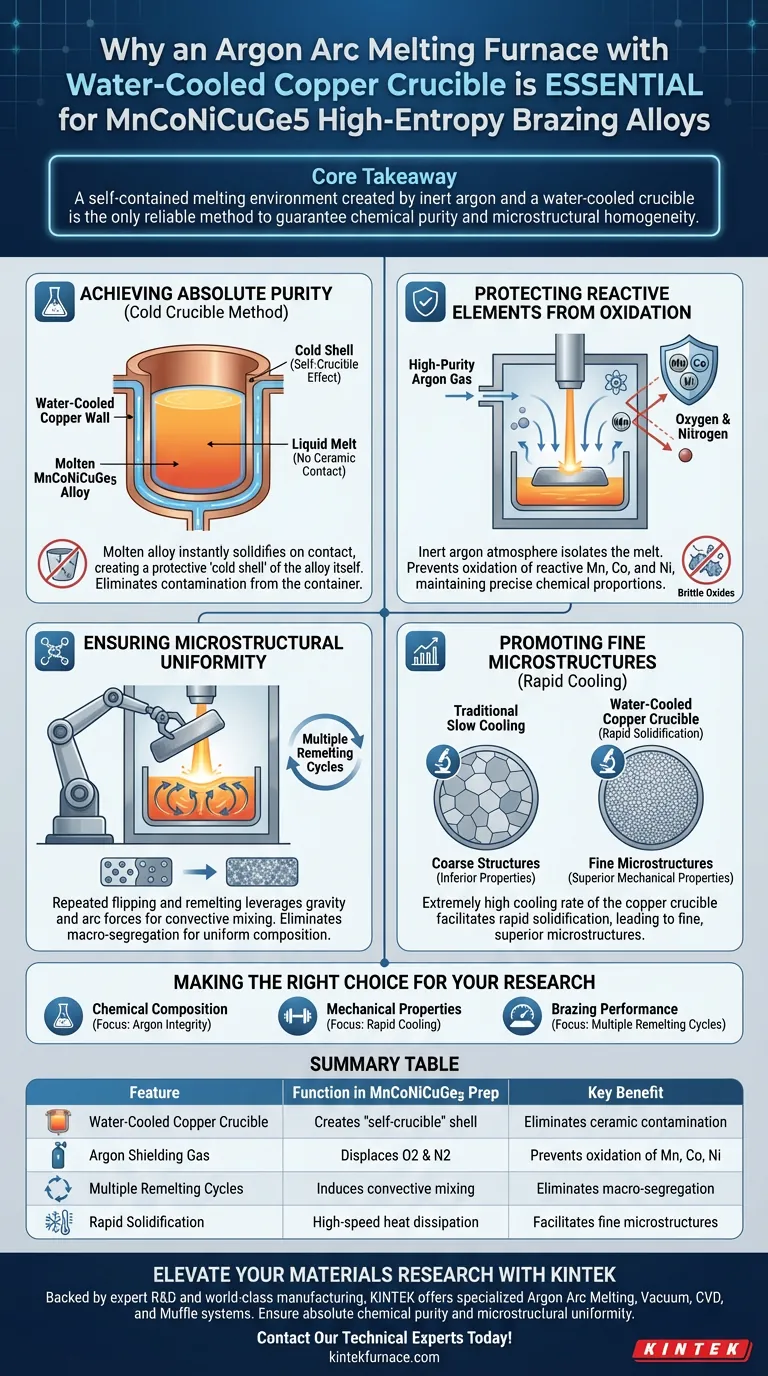
The preparation of MnCoNiCuGe5 high-entropy brazing alloys requires an argon arc melting furnace with a water-cooled copper crucible to guarantee chemical purity and microstructural homogeneity. This specific equipment setup is the only reliable method to melt reactive elements without introducing contaminants from the containment vessel or the atmosphere.
Core Takeaway: The combination of an inert argon atmosphere and a water-cooled crucible creates a "self-contained" melting environment. This prevents the alloy from reacting with oxygen or the crucible walls, ensuring the final material retains the precise chemical proportions necessary for high-quality brazing performance.
Achieving Absolute Purity via the Cold Crucible Method
The Self-Crucible Effect
The primary challenge in melting high-entropy alloys is preventing the molten metal from reacting with the container. Standard ceramic crucibles can introduce impurities at ultra-high temperatures.
The water-cooled copper crucible solves this through rapid heat dissipation. When the molten alloy contacts the water-cooled copper walls, it solidifies instantly. This creates a thin "cold shell" of the alloy itself, effectively acting as the container. The liquid melt resides inside this shell, never directly touching the copper, which eliminates contamination.
Avoiding Material Degradation
For the MnCoNiCuGe5 alloy, maintaining purity is critical for researching the microstructure of brazed joints. By utilizing the cold crucible technique, the process ensures that no foreign oxides or ceramic particles migrate into the melt. This provides a solid, contaminant-free foundation for analyzing the alloy's true properties.
Protecting Reactive Elements from Oxidation
Shielding Manganese, Cobalt, and Nickel
The alloy contains active elements—specifically manganese (Mn), cobalt (Co), and nickel (Ni)—that are highly prone to oxidation at melting temperatures.
High-purity argon gas serves as a protective atmosphere within the furnace. It effectively isolates the melt from oxygen and nitrogen in the surrounding air. Without this inert shield, these active elements would form brittle oxides or nitrides, deviating the alloy from its theoretical chemical proportions and ruining the wettability and fluidity required for brazing.
Ensuring Microstructural Uniformity
Eliminating Macro-Segregation
High-entropy alloys like MnCoNiCuGe5 consist of multiple principal elements that must be mixed perfectly. The argon arc furnace facilitates this through multiple remelting cycles.
By repeatedly flipping and re-melting the ingot, the equipment leverages gravity and arc forces to induce convective mixing. This mechanical agitation eliminates macro-segregation (separation of elements), ensuring that the chemical composition is uniform throughout the entire ingot.
Promoting Fine Microstructures
The cooling rate significantly impacts the final quality of the alloy. The water-cooled copper crucible provides an extremely high cooling rate compared to traditional methods. This rapid solidification facilitates the formation of fine solidified microstructures, which generally exhibit superior mechanical properties compared to coarse structures formed by slow cooling.
Understanding the Trade-offs
Process Sensitivity
While this method offers superior purity, it relies heavily on operator precision regarding process cycles. Achieving true homogeneity is not automatic; it requires a disciplined regimen of multiple flipping and remelting operations. If the number of cycles is insufficient, the complex mix of five elements (Mn, Co, Ni, Cu, Ge) may not achieve the necessary uniform distribution, rendering the sample unreliable for research.
Making the Right Choice for Your Research
To maximize the quality of your MnCoNiCuGe5 preparation, align your process with your specific experimental goals:
- If your primary focus is Chemical Composition: Prioritize the integrity of the argon atmosphere to prevent the loss of manganese and cobalt to oxidation.
- If your primary focus is Mechanical Properties: Ensure you utilize the rapid cooling capabilities of the copper crucible to generate a fine, uniform microstructure.
- If your primary focus is Brazing Performance: Strictly adhere to multiple remelting cycles to guarantee the homogeneity required for consistent fluidity and wetting.
Success in high-entropy alloy research depends not just on melting the metal, but on strictly controlling the thermal and chemical environment during the liquid phase.
Summary Table:
| Feature | Function in MnCoNiCuGe5 Preparation | Key Benefit |
|---|---|---|
| Water-Cooled Copper Crucible | Creates a "self-crucible" shell | Eliminates ceramic contamination and chemical impurities. |
| Argon Shielding Gas | Displaces oxygen and nitrogen | Prevents oxidation of reactive elements like Mn, Co, and Ni. |
| Multiple Remelting Cycles | Induces convective mixing | Eliminates macro-segregation for chemical homogeneity. |
| Rapid Solidification | High-speed heat dissipation | Facilitates fine microstructures and superior mechanical properties. |
Elevate Your Materials Research with KINTEK
Precision in high-entropy alloy (HEA) synthesis starts with the right thermal environment. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Argon Arc Melting, Vacuum, CVD, and Muffle systems designed to meet the rigorous demands of advanced metallurgy.
Whether you are developing MnCoNiCuGe5 brazing alloys or custom HEA compositions, our lab high-temperature furnaces are fully customizable to your unique research needs. Ensure absolute chemical purity and microstructural uniformity in every melt.
Ready to optimize your alloy preparation? Contact our technical experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- S.V. Maksymova, V.V. Voronov. Structure formation of seams using high-entropic brazing filler metal MnCoNiCuGe5. DOI: 10.21203/rs.3.rs-7260180/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the advantages of using a Vacuum Induction Melting Furnace over an ordinary open melting furnace? Achieve Purity and Precision in Metal Production
- What industries benefit from Vacuum Induction Melting Furnaces? Unlock High-Purity Metals for Aerospace, Medical, and More
- What precious metals can be melted in induction furnaces? Efficient, Clean Melting for Gold, Silver, and Platinum Group Metals
- What are the benefits of vacuum induction smelting technology? Achieve Superior Purity and Performance for Advanced Materials
- What are the primary functions of a Vacuum Induction Melting (VIM) furnace? Optimize Ni30 Superalloy Purity
- Why is precise temperature control in an electric melting furnace essential for AZ91D/Si3N4/WGP composites?
- What type of heating system is commonly used in vacuum casting furnaces and how does it work? Discover Induction Heating for Pure, Efficient Melting
- What are the key benefits of using an IGBT Vacuum Induction Melting Furnace? Achieve Superior Metal Purity and Control



















