The requirement for these systems is dictated by the thermodynamic instability of silicon nitride at high temperatures. Without a protective environment, silicon nitride will chemically degrade before it can sinter into a dense ceramic. A vacuum or high-purity inert gas system serves two critical functions: it creates the necessary partial pressure to stop the material from decomposing into raw silicon and nitrogen gas, and it eliminates oxygen to prevent the formation of unwanted silica.
Silicon nitride is thermodynamically unstable at sintering temperatures. A controlled atmosphere is mandatory to inhibit thermal decomposition and prevent oxidation, ensuring the material achieves the necessary density and phase transformation without chemical degradation.

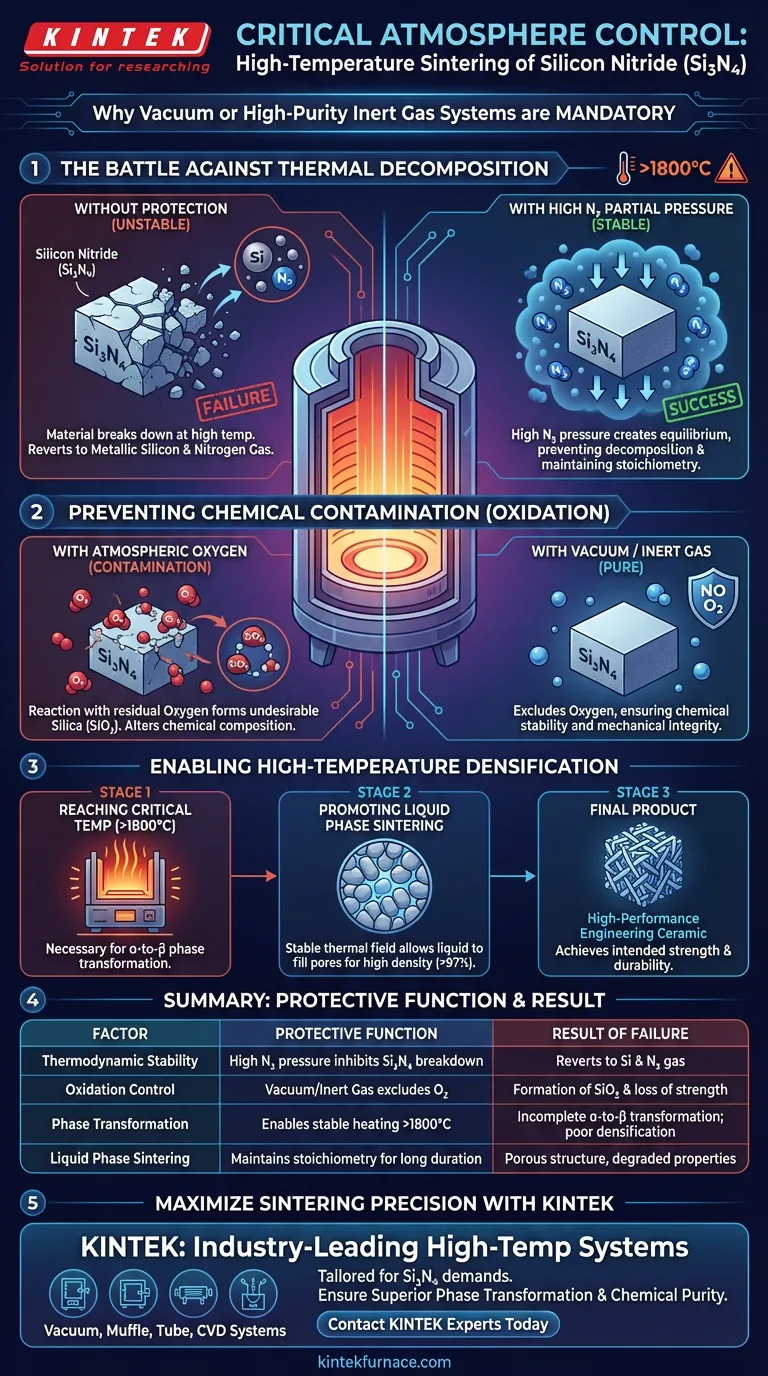
The Battle Against Thermal Decomposition
Understanding Material Instability
Silicon nitride ($Si_3N_4$) faces a fundamental challenge: it is thermodynamically unstable at the extreme temperatures required for sintering.
Without intervention, the material will undergo thermal decomposition. Instead of densifying, the ceramic bonds break, causing the material to revert into metallic silicon and nitrogen gas.
The Role of Partial Pressure
To counter this, you must introduce a specific atmosphere, typically high-purity nitrogen.
By maintaining a high partial pressure of nitrogen within the furnace, you effectively push back against the decomposition reaction. This pressure equilibrium forces the silicon nitride to remain in its compound state, maintaining the material's stoichiometry.
Preventing Chemical Contamination
The Risk of Oxidation
Beyond decomposition, the presence of atmospheric oxygen is a critical threat.
If exposed to residual oxygen at high heat, silicon nitride oxidizes to form silica ($SiO_2$). This reaction fundamentally alters the chemical composition of the ceramic.
Ensuring Mechanical Integrity
The formation of silica is detrimental to the high-temperature mechanical properties of the final product.
A vacuum or inert gas system excludes oxygen from the chamber. This ensures the chemical stability required to maintain the ceramic's intended strength and durability.
Enabling High-Temperature Densification
Reaching Critical Temperatures
High-performance silicon nitride often requires temperatures exceeding 1800°C to sinter correctly.
These extreme temperatures are necessary to drive the alpha-to-beta phase transformation, which creates the material's signature interlaced columnar crystal structure. A protected atmosphere allows the furnace to reach these temperatures without destroying the material.
Promoting Liquid Phase Sintering
Achieving high density (exceeding 97%) relies on a liquid phase formed by sintering aids.
The furnace must maintain a stable thermal field for extended periods (e.g., 120 minutes) to allow this liquid to fill pores. The protective gas environment ensures the base material remains stable throughout this long-duration insulation, allowing grain rearrangement and growth to proceed uninterrupted.
Understanding the Trade-offs
Temperature vs. Pressure Requirements
There is a direct correlation between temperature and the required gas pressure.
As sintering temperatures rise to accelerate densification, the thermodynamic drive for decomposition increases. Consequently, you must increase the nitrogen pressure (e.g., to 0.1 MPa or higher) to compensate and maintain stability.
Atmosphere Purity vs. Cost
Achieving "high purity" nitrogen or argon adds complexity and cost to the operation.
However, compromising on gas purity introduces oxygen. Even trace amounts can lead to surface oxidation or inconsistent mechanical properties, negating the benefits of the high-temperature process.
Making the Right Choice for Your Goal
To ensure the success of your sintering process, align your atmosphere control with your specific material objectives:
- If your primary focus is Structural Integrity: Prioritize maintaining sufficient nitrogen partial pressure to completely inhibit thermal decomposition into silicon and gas.
- If your primary focus is Chemical Purity: Ensure the system is capable of high-vacuum or uses ultra-high-purity gas to strictly exclude oxygen and prevent silica formation.
By precisely controlling the furnace atmosphere, you transform a thermodynamically unstable powder into a high-performance engineering ceramic.
Summary Table:
| Factor | Protective Function | Result of Failure |
|---|---|---|
| Thermodynamic Stability | High nitrogen partial pressure inhibits $Si_3N_4$ breakdown | Material reverts to metallic silicon and nitrogen gas |
| Oxidation Control | High vacuum or inert gas (Argon/Nitrogen) excludes $O_2$ | Formation of unwanted $SiO_2$ (silica) and loss of strength |
| Phase Transformation | Enables stable heating above 1800°C | Incomplete alpha-to-beta transformation; poor densification |
| Liquid Phase Sintering | Maintains stoichiometry for long-duration insulation | Porous ceramic structure with degraded mechanical properties |
Maximize Your Sintering Precision with KINTEK
Don’t let thermal decomposition or oxidation compromise your material integrity. KINTEK provides industry-leading high-temperature vacuum and atmospheric systems designed specifically for the rigorous demands of silicon nitride sintering.
Backed by expert R&D and world-class manufacturing, we offer customizable Vacuum, Muffle, Tube, and CVD systems tailored to your unique lab or production requirements. Ensure superior phase transformation and chemical purity in every batch.
Ready to optimize your high-performance ceramic production?
Visual Guide
References
- ESTIMATION OF VOLATILE MATTER, HEATING VALUE, POROXIMATE, ULTIMATE AND STRUCTURAL COMPOSITION OF BIOMASS (ELEPHANT GRASS). DOI: 10.56726/irjmets48152
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role do the exhaust branch pipes at the top of a vacuum chamber play? Optimize Your Pressure Control Today
- What is the core technical mechanism of low-oxygen brazing? Master Oxide Decomposition for Perfect Bonds
- What core role does a high-temperature vacuum sintering furnace play in Sm:YAG ceramics? Mastering Optical Clarity
- What are the advantages of using a laboratory vacuum drying oven? Essential Benefits for Graphene Composite Powders
- How does a laboratory vacuum high-temperature furnace maintain conditions for LiF-BeF2-LaF3? Expert Atmosphere Control
- How does a vacuum furnace work to prevent metal oxidation? Achieve Purity in High-Temperature Metal Processing
- How does a vacuum furnace improve material purity? Achieve Superior Material Integrity with Controlled Environments
- How does the combined use of a centrifuge and a vacuum drying oven solve issues in H-Beta zeolite catalyst recovery?



















