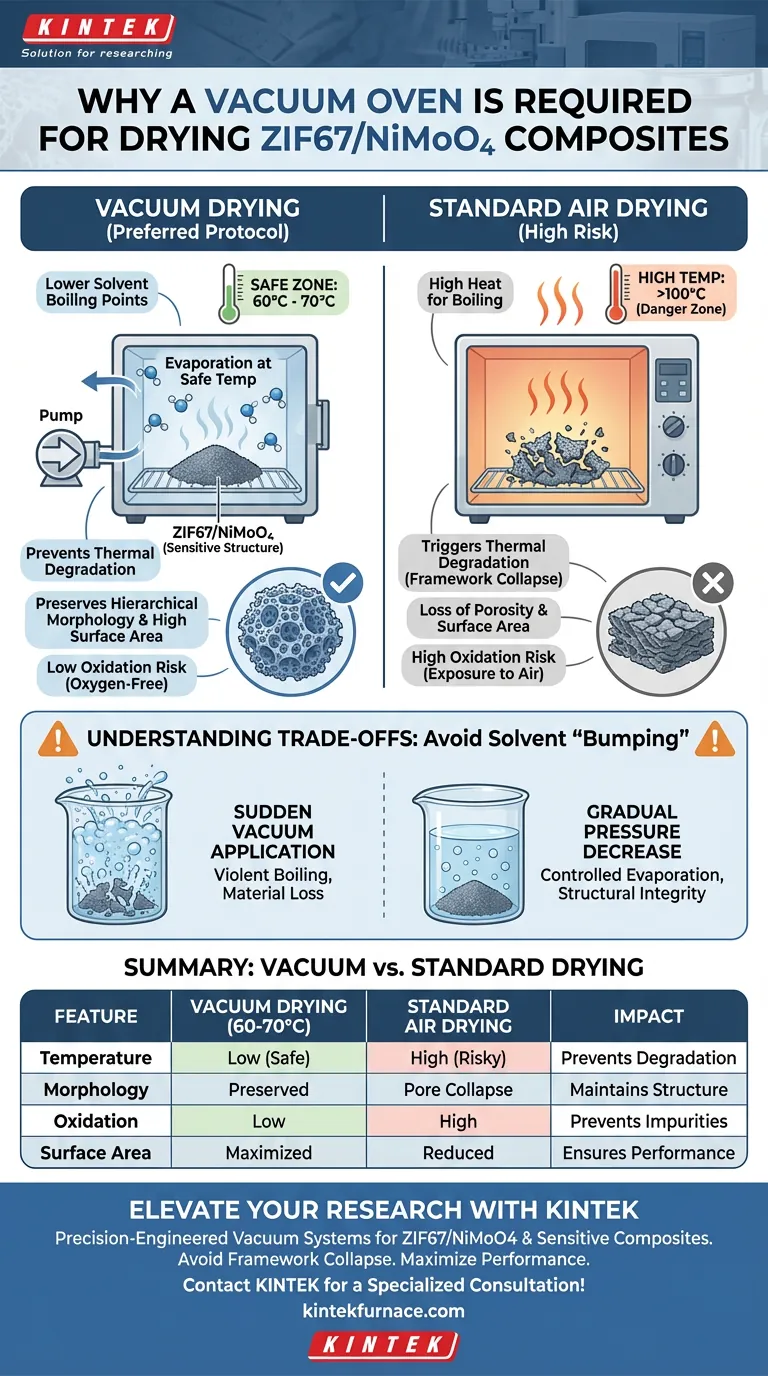
The primary requirement for using a vacuum oven with ZIF67/NiMoO4 composites is to facilitate the rapid removal of solvents at significantly reduced temperatures, typically between 60°C and 70°C. Because ZIF67 is structurally sensitive, the vacuum environment is essential to lower the boiling point of volatiles, allowing for thorough drying without subjecting the material to the high heat that causes framework collapse.
By reducing ambient pressure, a vacuum oven allows solvents to evaporate without reaching temperatures that trigger thermal degradation. This process is critical for preserving the hierarchical morphology and high specific surface area that determine the composite's performance.

The Critical Role of Temperature Control
Avoiding Thermal Degradation
ZIF67 (Zeolitic Imidazolate Framework-67) and its composites are often thermally instable.
Subjecting these materials to standard high-temperature drying can break the chemical bonds within the framework. By utilizing a vacuum, you can remove moisture effectively within a safe thermal window (60–70°C), preventing the material from degrading.
Lowering Solvent Boiling Points
Under standard atmospheric pressure, removing solvents often requires heat that exceeds the stability limit of the composite.
The vacuum environment significantly lowers the boiling point of water and other solvents. This physical change allows volatiles to turn into gas and evacuate the material rapidly, even at modest temperatures.
Preserving Material Architecture
Maintaining Hierarchical Morphology
The effectiveness of ZIF67/NiMoO4 is heavily dependent on its physical structure.
Conventional drying methods can cause capillary forces or thermal stress that lead to pore collapse. Vacuum drying is a gentler process that preserves the intricate, hierarchical morphology of the composite.
Protecting Specific Surface Area
High specific surface area is a key performance metric for these composites.
If the framework collapses due to heat, active sites become inaccessible. Vacuum drying ensures the pores remain open and the surface area is maximized for future chemical reactions or electrochemical applications.
Understanding the Trade-offs
The Risk of Solvent "Bumping"
While vacuum drying is efficient, applying the vacuum too suddenly can cause solvents to boil violently.
This phenomenon, known as "bumping," can physically disrupt the powder or cause material loss. It is essential to decrease pressure gradually to allow for controlled evaporation.
Oxidation Prevention
While the primary reference highlights structural preservation, supplementary data suggests a secondary benefit: oxidation control.
High-temperature air drying can lead to secondary oxidation of metallic components. A vacuum environment removes oxygen, adding a layer of chemical protection to the structural benefits.
Making the Right Choice for Your Goal
To ensure the highest quality synthesis of ZIF67/NiMoO4, align your drying protocol with your specific performance targets:
- If your primary focus is Structural Integrity: strict adherence to the 60–70°C range under vacuum is required to prevent framework collapse and loss of surface area.
- If your primary focus is Chemical Purity: ensure your vacuum system has high seal integrity to eliminate oxygen, preventing secondary oxidation during the heating phase.
Using a vacuum oven is not merely a method of speeding up the process; it is a fundamental requirement for retaining the functional properties of temperature-sensitive MOF composites.
Summary Table:
| Feature | Vacuum Drying (60-70°C) | Standard Air Drying | Impact on ZIF67/NiMoO4 |
|---|---|---|---|
| Temperature | Low (Safe for MOFs) | High (Required for boiling) | Prevents thermal degradation |
| Morphology | Preserved | Risk of pore collapse | Maintains hierarchical structure |
| Oxidation | Low (Oxygen-free) | High | Prevents chemical impurities |
| Surface Area | Maximized | Reduced | Ensures high electrochemical performance |
Elevate Your Material Research with KINTEK
Preserving the integrity of sensitive composites like ZIF67/NiMoO4 requires precision-engineered thermal equipment. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Vacuum, Muffle, Tube, Rotary, and CVD systems tailored to your laboratory's exacting standards.
Whether you need customizable temperature windows or superior vacuum integrity to prevent framework collapse and oxidation, our team is ready to design a solution for your unique synthesis needs. Maximize your material's surface area and performance today—Contact KINTEK for a specialized consultation!
Visual Guide
References
- Kandasamy Sasikumar, Heongkyu Ju. Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. DOI: 10.3390/ma17246225
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 1200℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What function does a High-Pressure Hydrogen Annealing Furnace serve? Achieving Deep Saturation in Steel Samples
- What are the tool and die industry applications of furnace brazing? Boost Performance and Cut Costs
- Why is a vacuum high-temperature furnace necessary for Cu-Cr-Zr-La alloy ingots? Ensure Material Uniformity
- Why is a high vacuum system necessary when using SPS for Ti-6Al-4V composites? Ensure Material Integrity
- What is the primary function of a vacuum arc melting furnace? Expert Solutions for High-Entropy Alloy Production
- What are the key benefits of using a vacuum sintering furnace? Achieve Superior Material Purity and Process Control
- What are the key components of a vacuum sintering furnace? Essential Parts for Precision Material Processing
- How do vacuum sintering furnaces compare to traditional furnaces? Unlock Superior Material Quality and Control



















