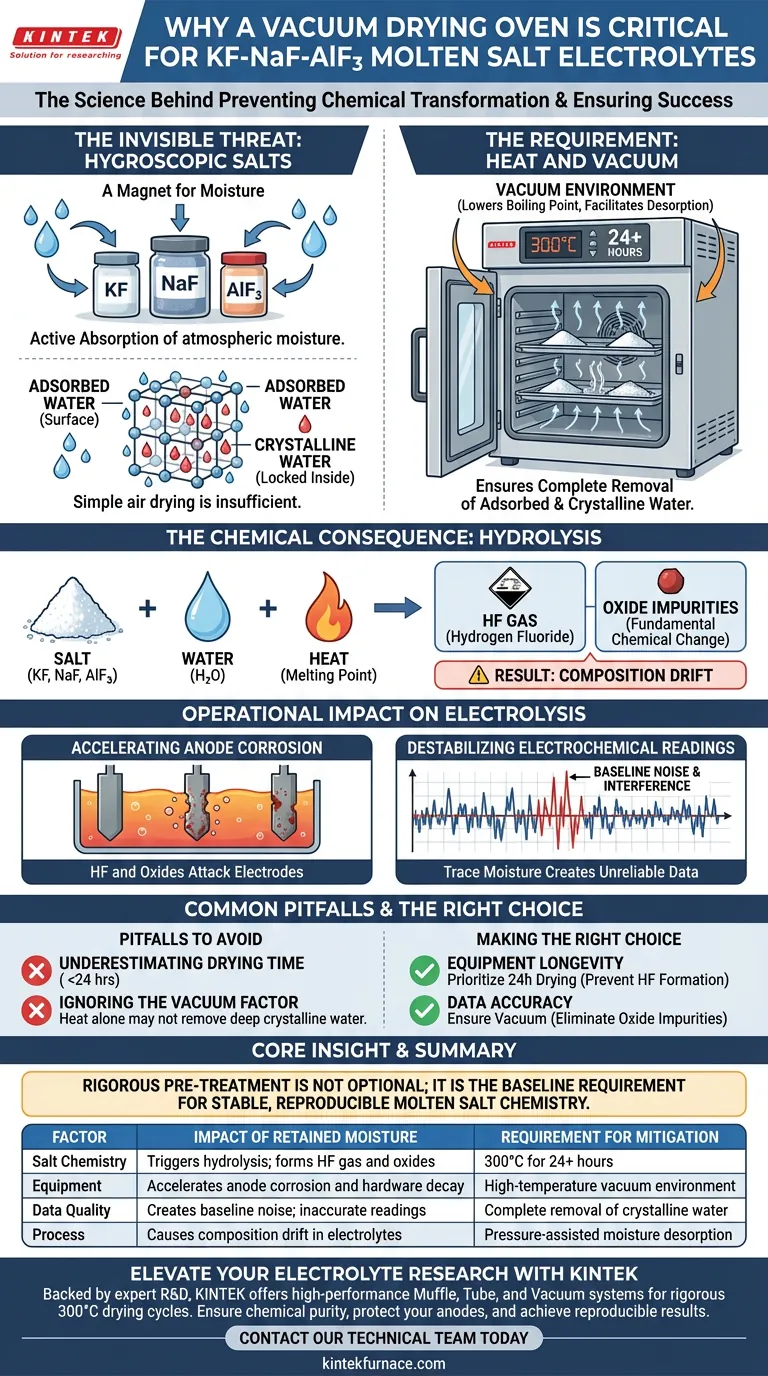
The necessity of a vacuum drying or high-temperature oven stems directly from the intense hygroscopic nature of fluoride salts like KF, NaF, and AlF3. Without rigorous drying at 300°C for at least 24 hours, retained moisture triggers hydrolysis reactions that fundamentally alter the electrolyte's chemistry and degrade system hardware.
Core Insight: The goal of drying is not merely to remove wetness, but to prevent a chemical transformation. Moisture left in fluoride salts during melting creates hydrofluoric acid and oxide impurities, leading to severe anode corrosion and unreliable electrochemical data.

The Invisible Threat: Hygroscopic Salts
A Magnet for Moisture
The components of your electrolyte—specifically Potassium Fluoride (KF), Sodium Fluoride (NaF), and Aluminum Fluoride (AlF3)—are highly hygroscopic.
This means they actively absorb moisture from the surrounding atmosphere.
Adsorbed vs. Crystalline Water
This moisture exists in two forms: physically adsorbed water on the surface and crystalline water locked within the salt structure.
Simple air drying is insufficient to remove these tightly bound water molecules.
The Requirement for Heat and Vacuum
To ensure complete removal, the materials must be processed at 300°C for a minimum of 24 hours.
Using a vacuum environment enhances this process by lowering the boiling point of water and facilitating the desorption of moisture from the salt's pores.
The Chemical Consequence: Hydrolysis
Turning Salt into Acid
If moisture remains present when the salts are heated to their melting point, a hydrolysis reaction occurs.
The water reacts with the fluoride salts to generate Hydrogen Fluoride (HF) gas.
Formation of Oxide Impurities
Simultaneously, this reaction converts pure fluorides into oxides (impurities).
This fundamentally changes the chemical composition of your molten salt, leading to "composition drift" where the ratio of electrolyte components is no longer what you calculated.
Operational Impact on Electrolysis
Accelerating Anode Corrosion
The presence of moisture and the resulting oxides is a primary cause of anode corrosion.
These impurities attack the electrode materials, degrading them rapidly and contaminating the melt further with electrode byproducts.
Destabilizing Electrochemical Readings
Trace moisture interferes with the electrochemical baseline.
Impurity ions create noise in the reduction waveforms, making it difficult to distinguish the true signal of target metals (like niobium or titanium) from background interference.
Common Pitfalls to Avoid
Underestimating Drying Time
A common mistake is reducing the drying time below 24 hours to speed up production.
Even small amounts of residual moisture can trigger enough hydrolysis to ruin a batch of electrolyte.
Ignoring the Vacuum Factor
While high heat is effective, heat alone may not remove trace moisture trapped deep within the salt's crystal lattice.
The vacuum pressure is the mechanical force that pulls these final trace amounts out of the material.
Making the Right Choice for Your Goal
To ensure the success of your molten salt process, apply the following principles:
- If your primary focus is Equipment Longevity: Prioritize the 24-hour drying cycle to prevent HF formation, which aggressively corrodes oven interiors and anodes.
- If your primary focus is Data Accuracy: Ensure a vacuum environment is used to eliminate oxide impurities that cause baseline noise and inaccurate electrochemical readings.
Rigorous pre-treatment is not an optional step; it is the baseline requirement for stable, reproducible molten salt chemistry.
Summary Table:
| Factor | Impact of Retained Moisture | Requirement for Mitigation |
|---|---|---|
| Salt Chemistry | Triggers hydrolysis; forms HF gas and oxides | 300°C for 24+ hours |
| Equipment | Accelerates anode corrosion and hardware decay | High-temperature vacuum environment |
| Data Quality | Creates baseline noise; inaccurate readings | Complete removal of crystalline water |
| Process | Causes composition drift in electrolytes | Pressure-assisted moisture desorption |
Elevate Your Electrolyte Research with KINTEK
Don't let trace moisture compromise your electrochemical data or destroy your hardware. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, and Vacuum systems designed specifically for the rigorous 300°C drying cycles required for KF-NaF-AlF3 salts. Whether you need a standard solution or a system customized for your unique lab requirements, our high-temp furnaces ensure the chemical purity your process demands.
Ready to protect your anodes and ensure reproducible results? Contact our technical team today!
Visual Guide
References
- Kamaljeet Singh, Guðrún Sævarsdóttir. Overpotential on Oxygen-Evolving Platinum and Ni-Fe-Cu Anode for Low-Temperature Molten Fluoride Electrolytes. DOI: 10.1007/s11837-024-06425-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a high vacuum system critical for sealing the quartz tube used in Fe3GeTe2 single crystal preparation?
- How do thermal imagers and hybrid AI models facilitate leak detection? 92% Accuracy in Industrial Furnaces
- What are the overall advantages of vacuum furnaces? Achieve Purity, Precision, and Repeatability
- How does a vacuum heat treatment furnace prevent contamination? Ensure Purity in High-Temperature Processes
- What is vacuum annealing and what benefits does it provide? Achieve Superior Material Properties and Pristine Surfaces
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- Why is prolonged treatment in a vacuum drying oven necessary for SnO2-based anodes? Ensure Reliable Electrochemical Data
- What are the technical advantages of using a high-vacuum high-temperature sintering furnace for stainless steel?



















