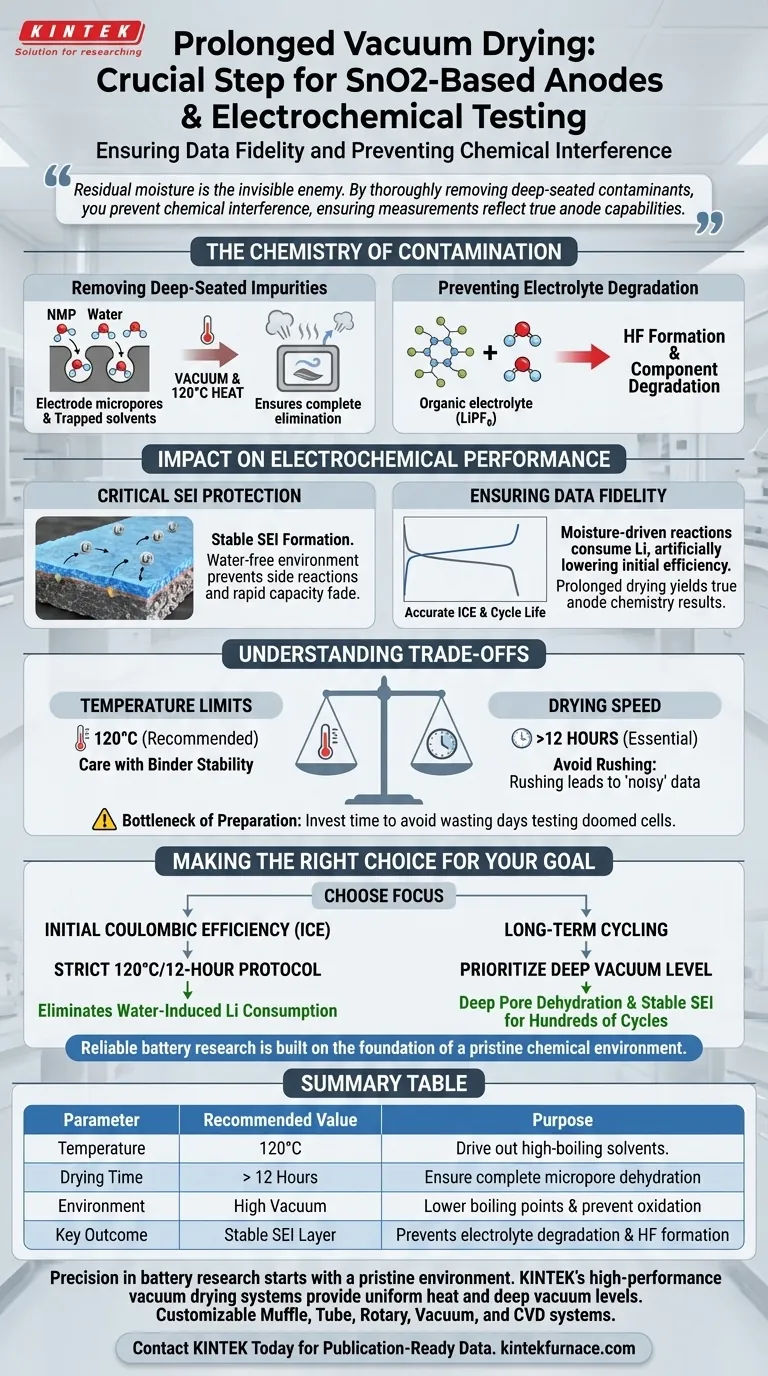
Prolonged vacuum drying is the critical preparatory step required to eliminate trace moisture and residual solvents that otherwise compromise electrochemical data. For SnO2-based anodes, treating electrode sheets at 120°C for over 12 hours under high vacuum ensures that residual water molecules do not trigger parasitic reactions, thereby preserving the integrity of your test results.
Residual moisture is the invisible enemy of battery chemistry. By thoroughly removing deep-seated contaminants, you prevent chemical interference with the electrolyte, ensuring that your measurements reflect the true capabilities of the anode material rather than the artifacts of contamination.

The Chemistry of Contamination
Removing Deep-Seated Impurities
During the electrode manufacturing process, solvents such as N-Methyl-2-pyrrolidone (NMP) are often used to create the slurry.
Even after the initial drying phase, trace amounts of these solvents and environmental moisture remain trapped within the micropores of the electrode material.
A simple air-dry is insufficient; high vacuum lowers the boiling point of these liquids, while the 120°C heat provides the energy to drive them out completely.
Preventing Electrolyte Degradation
The organic electrolytes used in lithium-ion batteries are highly sensitive to water.
If moisture remains in the anode, it reacts with the lithium salts (such as LiPF6) present in the electrolyte.
This reaction can produce harmful byproducts, such as hydrofluoric acid (HF), which actively degrades the cell components before testing even begins.
Impact on Electrochemical Performance
Critical Protection of the SEI Layer
The formation of the Solid Electrolyte Interphase (SEI) layer during the first cycle is the most important factor in a battery's longevity.
Water molecules trapped in the anode undergo side reactions that disrupt the formation of a stable SEI.
An unstable SEI leads to continuous electrolyte consumption and rapid capacity fade, making the anode appear less stable than it actually is.
Ensuring Data Fidelity
To evaluate an SnO2-based anode, you must isolate its performance from external variables.
Moisture-driven side reactions consume lithium, artificially lowering your initial charge-discharge efficiency.
Prolonged drying ensures that the data you collect—specifically regarding efficiency and cycle life—is a result of the anode chemistry, not contamination.
Understanding the Trade-offs
Temperature Limits vs. Drying Speed
While the primary recommendation for SnO2 is 120°C, you must be mindful of your binder material.
Some polymer binders may degrade or become brittle if the temperature exceeds their thermal stability limits.
However, lowering the temperature (e.g., to 60°C) generally requires significantly longer drying times to achieve the same level of moisture removal.
The Bottleneck of Preparation
The requirement for over 12 hours of drying time can create a workflow bottleneck in high-throughput testing.
Attempting to rush this step is a common pitfall that results in "noisy" data and poor reproducibility.
It is always more efficient to spend the extra time drying than to waste days testing a cell that was doomed by moisture from the start.
Making the Right Choice for Your Goal
To ensure your electrochemical testing yields publication-quality data, apply the following principles:
- If your primary focus is Initial Coulombic Efficiency (ICE): Adhere strictly to the 120°C/12-hour protocol to eliminate water-induced lithium consumption.
- If your primary focus is Long-Term Cycling: Prioritize the vacuum level to ensure deep pore dehydration, which is essential for a stable SEI layer over hundreds of cycles.
Reliable battery research is built on the foundation of a pristine chemical environment.
Summary Table:
| Parameter | Recommended Value | Purpose |
|---|---|---|
| Temperature | 120°C | Drive out high-boiling solvents like NMP |
| Drying Time | > 12 Hours | Ensure complete dehydration of micropores |
| Environment | High Vacuum | Lower boiling points & prevent oxidation |
| Key Outcome | Stable SEI Layer | Prevents electrolyte degradation and HF formation |
Precision in battery research starts with a pristine environment. KINTEK’s high-performance vacuum drying systems are engineered to provide the uniform heat and deep vacuum levels necessary for critical anode preparation. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your laboratory's unique high-temperature needs. Contact KINTEK today to discover how our equipment can eliminate chemical interference and ensure your electrochemical data is publication-ready.
Visual Guide
References
- Antunes Staffolani, Francesco Nobili. Tailoring the Electrochemical Performance of SnO<sub>2</sub>‐Based Anodes for Li‐Ion Batteries: Effect of Morphology and Composite Matrix. DOI: 10.1002/admt.202402058
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- Can you describe a process example using a vacuum hardening furnace? Achieve Clean, Precise Metal Hardening
- What role does vacuum annealing play in preventing material oxidation? Ensure Purity with Oxygen-Free Heat Treatment
- What is the vacuum heat treatment process? Achieve Superior Surface Quality and Material Performance
- Why is the drying step of the graphite furnace program necessary? Prevent Spattering for Accurate Results
- Why are the materials used in vacuum furnace construction critical? Ensure Peak Performance and Purity
- How does vacuum hardening affect the hardness and surface layer of high-alloy tool steel? Boost Performance with Superior Heat Treatment
- What are the key uses of vacuum annealing furnaces? Achieve Superior Material Integrity and Purity
- What are the benefits of using a vacuum atmosphere in metal melting? Achieve Ultimate Purity and Control



















