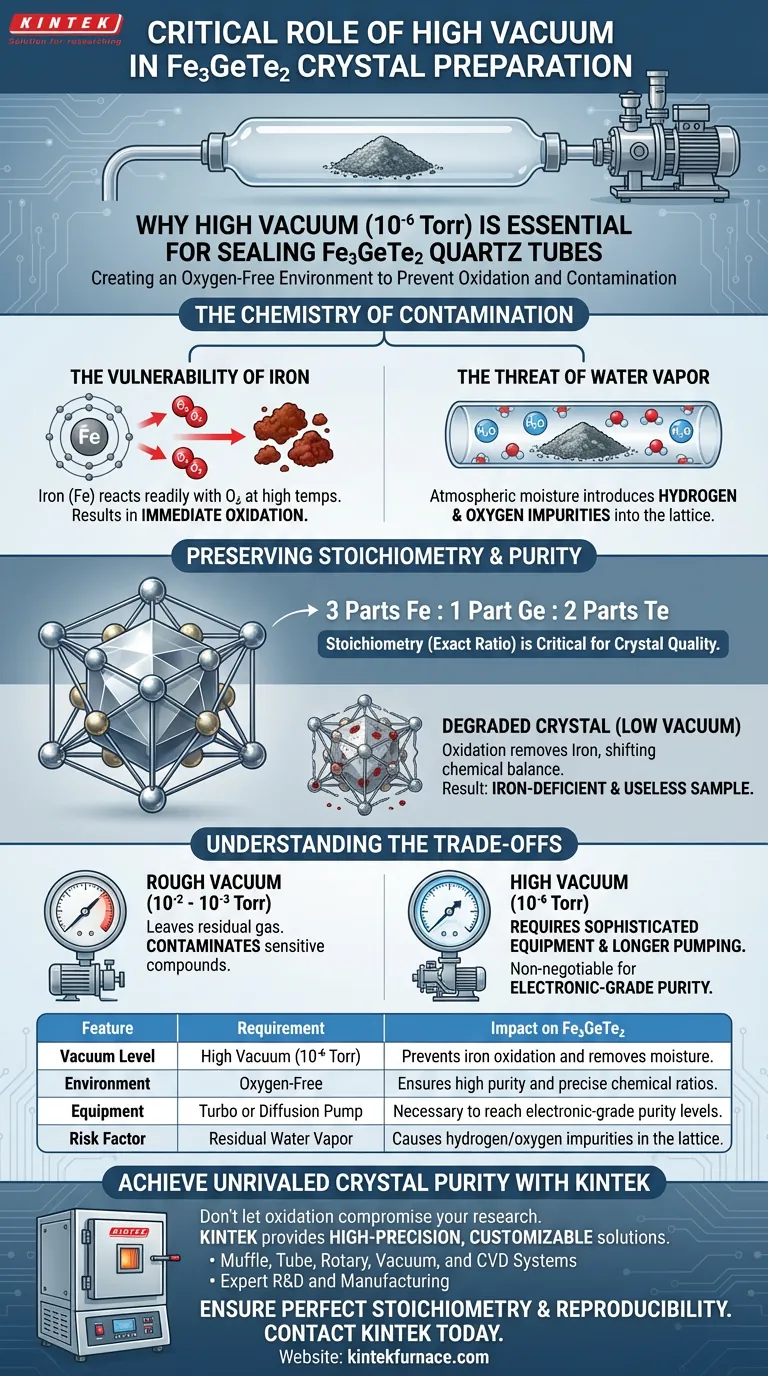
The primary function of a high vacuum system in Fe3GeTe2 preparation is to create an oxygen-free environment by evacuating the quartz tube to approximately 10⁻⁶ Torr.
This specific pressure level is required to completely remove air and water vapor before the tube is sealed. Because iron-based materials like Fe3GeTe2 are chemically aggressive and highly susceptible to oxidation at high temperatures, this vacuum step is the only way to prevent the raw materials from degrading during crystal growth.
The success of Fe3GeTe2 crystal growth hinges on the purity of the reaction environment. High vacuum prevents the formation of oxides, ensuring the final crystal retains the precise chemical ratio (stoichiometry) and high purity necessary for its intended properties.

The Chemistry of Contamination
The Vulnerability of Iron
Fe3GeTe2 contains iron (Fe), a transition metal that reacts readily with oxygen, especially when heated.
If air remains in the tube, the high temperatures required for crystal growth will cause the iron to oxidize immediately.
The Threat of Water Vapor
It is not just oxygen that poses a threat; atmospheric moisture is equally damaging.
A high vacuum system is essential to strip water vapor from the inner walls of the quartz and the raw materials themselves.
If water vapor remains, it can react with the starting materials, introducing hydrogen and oxygen impurities into the lattice.
Preserving Stoichiometry
Defining Stoichiometry
Stoichiometry refers to the exact numerical ratio of elements in a compound—in this case, 3 parts Iron, 1 part Germanium, and 2 parts Tellurium.
Crystal quality depends entirely on maintaining this specific "recipe" throughout the melting and cooling process.
Consequences of Oxidation
When iron oxidizes, it is effectively removed from the reaction pool intended for the crystal.
This shifts the chemical balance, leaving you with a crystal that is iron-deficient.
The result is a sample with degraded physical properties and significant impurities, rendering it useless for precise scientific study.
Understanding the Trade-offs
High Vacuum vs. Rough Vacuum
There is a distinct difference between a "rough" vacuum and the high vacuum (10⁻⁶ Torr) required here.
A simple rotary pump might achieve 10⁻² or 10⁻³ Torr, but this leaves enough residual gas to contaminate sensitive iron-based compounds.
The Cost of Precision
Achieving 10⁻⁶ Torr requires more sophisticated equipment, such as diffusion or turbo-molecular pumps, and longer pumping times.
While this adds complexity and duration to the preparation process, it is a non-negotiable cost for achieving electronic-grade purity in Fe3GeTe2.
Making the Right Choice for Your Goal
To ensure your single crystal preparation yields usable results, apply the following guidelines:
- If your primary focus is Crystal Purity: Ensure your system can reliably hold 10⁻⁶ Torr or lower; any leak or insufficient pumping will result in oxide inclusions.
- If your primary focus is Reproducibility: Standardize your evacuation time to ensure water vapor is consistently removed from the quartz walls in every batch.
The vacuum seal is not merely a container; it is the chemical gatekeeper that defines the ultimate quality of your material.
Summary Table:
| Feature | Requirement | Impact on Fe3GeTe2 |
|---|---|---|
| Vacuum Level | High Vacuum (10⁻⁶ Torr) | Prevents iron oxidation and removes moisture. |
| Environment | Oxygen-Free | Ensures high purity and precise chemical ratios. |
| Equipment | Turbo or Diffusion Pump | Necessary to reach electronic-grade purity levels. |
| Risk Factor | Residual Water Vapor | Causes hydrogen/oxygen impurities in the lattice. |
Achieve Unrivaled Crystal Purity with KINTEK
Don't let oxidation compromise your research. KINTEK provides high-precision high-temperature solutions specifically designed for sensitive material synthesis. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your unique laboratory needs.
Ensure perfect stoichiometry and reproducibility in every batch—Contact KINTEK today to consult with our experts on the ideal vacuum furnace for your Fe3GeTe2 preparation.
Visual Guide
References
- Microthermoreflectance Characterization of the Band‐Structure Transformations Observed During the Magnetic‐Ordering Transitions of Multilayered 2D Fe <sub>3</sub> GeTe <sub>2</sub> Ferromagnetic Metals. DOI: 10.1002/smsc.202500293
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the environmental advantages of vacuum-environment furnaces for coking? Learn how to eliminate leakage.
- What is the role of temperature control systems in a vacuum furnace? Ensure Precision in Heat Treatment Processes
- What are the advantages of using a vacuum heat treatment furnace? Achieve Superior Material Quality and Control
- How does the cooling system in a vacuum annealing furnace work? Master Efficient Heat Treatment for Your Materials
- How does the two-stage heat treatment in a vacuum sintering furnace optimize HA/Ti scaffolds? Master the Fabrication Process
- What is the function of a heating furnace in the distillation separation process of a High Vacuum Unit (HVU)?
- What benefits does vacuum heat treatment provide over traditional methods? Achieve Superior Material Quality and Control
- What is the core function of a vertical vacuum furnace in purifying crude magnesium? Master Precision Vacuum Distillation



















