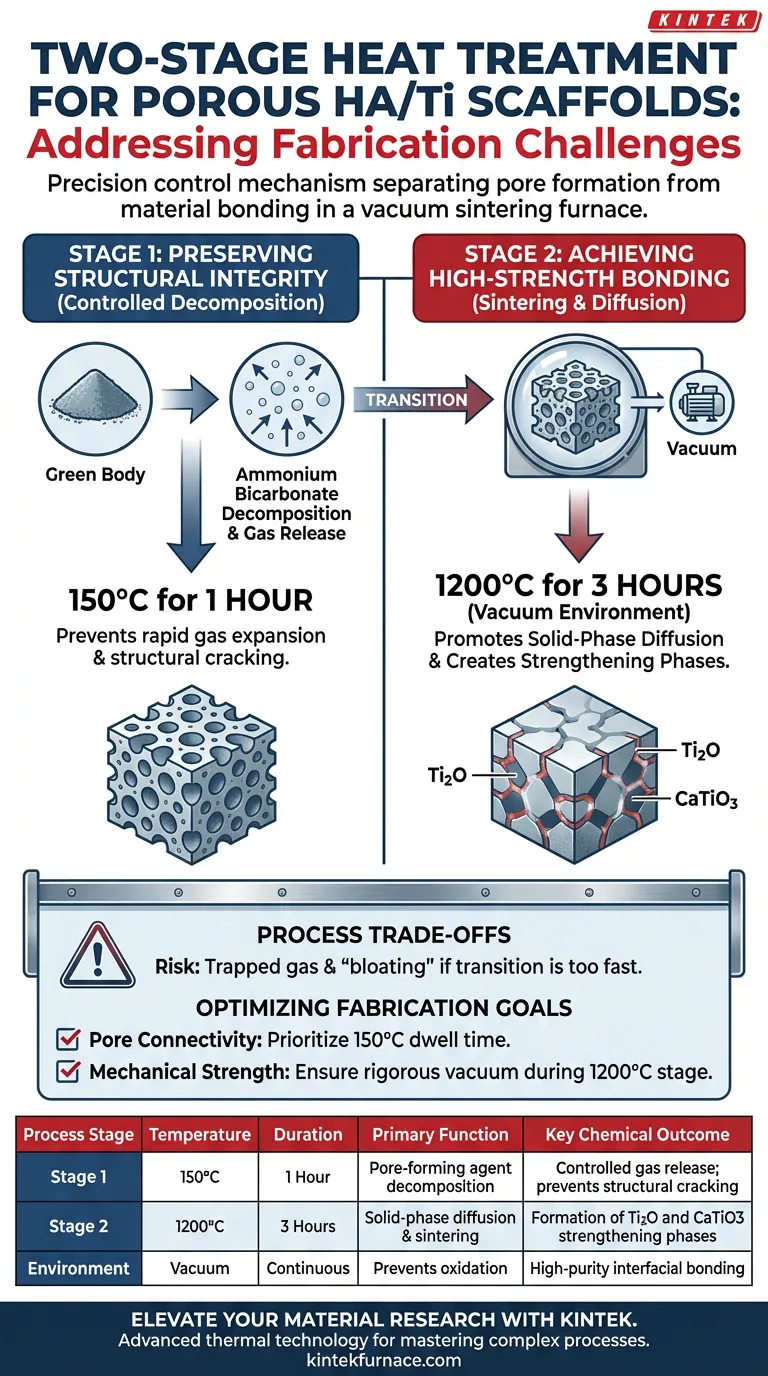
The two-stage heat treatment process acts as a precision control mechanism to separates the volatile removal of pore-forming agents from the high-energy demands of material bonding. This method addresses the dual challenge of preventing structural collapse during gas release (Stage 1 at 150°C) while ensuring robust interfacial strength through chemical phase transformation (Stage 2 at 1200°C).
Successful scaffold fabrication requires decoupling the mechanical stress of pore formation from the chemical process of sintering. This split approach prevents the green body from fracturing during gas expansion while creating the necessary conditions for strengthening phases like Ti2O and CaTiO3 to form.

Stage 1: Preserving Structural Integrity
The initial challenge in fabricating porous HA/Ti scaffolds is managing the removal of the pore-forming agent without destroying the delicate "green body" (the compacted but unfired powder structure).
The Risk of Rapid Gas Expansion
When pore-forming agents like ammonium bicarbonate decompose, they release gas. If this reaction occurs too rapidly or at too high a temperature, the internal pressure can shatter the scaffold before it has any mechanical strength.
Controlled Thermal Decomposition
The first stage addresses this by holding the temperature at 150°C for 1 hour. This specific thermal shelf allows for the slow, controlled decomposition of ammonium bicarbonate.
By moderating the rate of gas release, the process creates the desired porosity without inducing micro-cracks or catastrophic structural failure in the scaffold.
Stage 2: Achieving High-Strength Bonding
Once the pore structure is stabilized, the second challenge is transforming loose powder into a cohesive, load-bearing material. This requires significantly higher energy to trigger chemical changes.
Promoting Solid-Phase Diffusion
The second stage ramps the temperature to 1200°C for 3 hours within a vacuum environment. This high-temperature soak is essential to activate solid-phase diffusion, where atoms move between the Titanium and Hydroxyapatite (HA) particles.
Creating Strengthening Phases
The vacuum sintering process drives specific interfacial reactions that are impossible at lower temperatures. It facilitates the formation of new chemical compounds, specifically Ti2O and CaTiO3.
These new phases act as a metallurgical "glue." They provide high-strength bonding between the ceramic (HA) and metal (Ti) components, ensuring the scaffold can withstand mechanical loads.
Understanding the Process Trade-offs
While this two-stage process is effective, it introduces specific constraints that must be managed to avoid fabrication errors.
The Cost of Thermal Separation
The primary trade-off is the strict requirement for distinct thermal zones. Rushing from the decomposition phase (Stage 1) to the sintering phase (Stage 2) creates a conflict between gas evacuation and material shrinkage.
If the transition is too fast, residual gas becomes trapped inside the densifying material. This leads to internal defects or "bloating," which compromises the final strength provided by the Ti2O and CaTiO3 phases.
Optimizing Fabrication for Your Goals
To maximize the effectiveness of this heat treatment, align your process controls with your specific performance targets.
- If your primary focus is Pore Connectivity and Shape: Prioritize strict adherence to the 150°C dwell time, ensuring the ammonium bicarbonate is fully evacuated to prevent structural distortion.
- If your primary focus is Mechanical Strength and Durability: Ensure the vacuum environment is maintained rigorously during the 1200°C stage, as oxygen contamination or insufficient time will inhibit the formation of the vital Ti2O and CaTiO3 bonding layers.
By strictly segregating gas removal from material bonding, you transform a fragile powder compact into a robust, biomedically viable scaffold.
Summary Table:
| Process Stage | Temperature | Duration | Primary Function | Key Chemical Outcome |
|---|---|---|---|---|
| Stage 1 | 150°C | 1 Hour | Pore-forming agent decomposition | Controlled gas release; prevents structural cracking |
| Stage 2 | 1200°C | 3 Hours | Solid-phase diffusion & sintering | Formation of Ti2O and CaTiO3 strengthening phases |
| Environment | Vacuum | Continuous | Prevents oxidation | High-purity interfacial bonding |
Elevate Your Material Research with KINTEK
Precision is the difference between a fragile green body and a high-performance scaffold. KINTEK provides the advanced thermal technology necessary to master complex processes like two-stage vacuum sintering.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are fabricating biomedical HA/Ti scaffolds or advanced ceramics, our lab high-temp furnaces are fully customizable to meet your unique thermal profiles and atmosphere requirements.
Ready to optimize your sintering results?
Contact KINTEK today to discuss your project needs
Visual Guide
References
- Xingping Fan, Hao Zhang. Fabrication and Characterization of LaF3-Reinforced Porous HA/Ti Scaffolds. DOI: 10.3390/coatings14010111
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How do industrial-grade vacuum furnaces refine grain and relieve stress in Inconel 718? Achieve Peak Superalloy Strength
- What is the function of a vacuum drying oven in MAPbBr3@SiO2/PVDF preparation? Enhance Composite Stability & Density
- How does a vacuum environment affect heat transfer? Optimize Lithium Battery Drying with Conduction Mastery
- What are some common industrial uses of vacuum furnaces? Enhance Material Quality and Performance
- Why might a vacuum furnace maintain vacuum during cooling? Protect Workpieces from Oxidation and Control Metallurgy
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing
- What role do laboratory high-temperature furnaces play in the growth of Ni3In2Se2 single crystals? Precision Growth Control
- How does the low-pressure environment of an RH vacuum refining furnace influence the morphology of a supersonic jet?



















