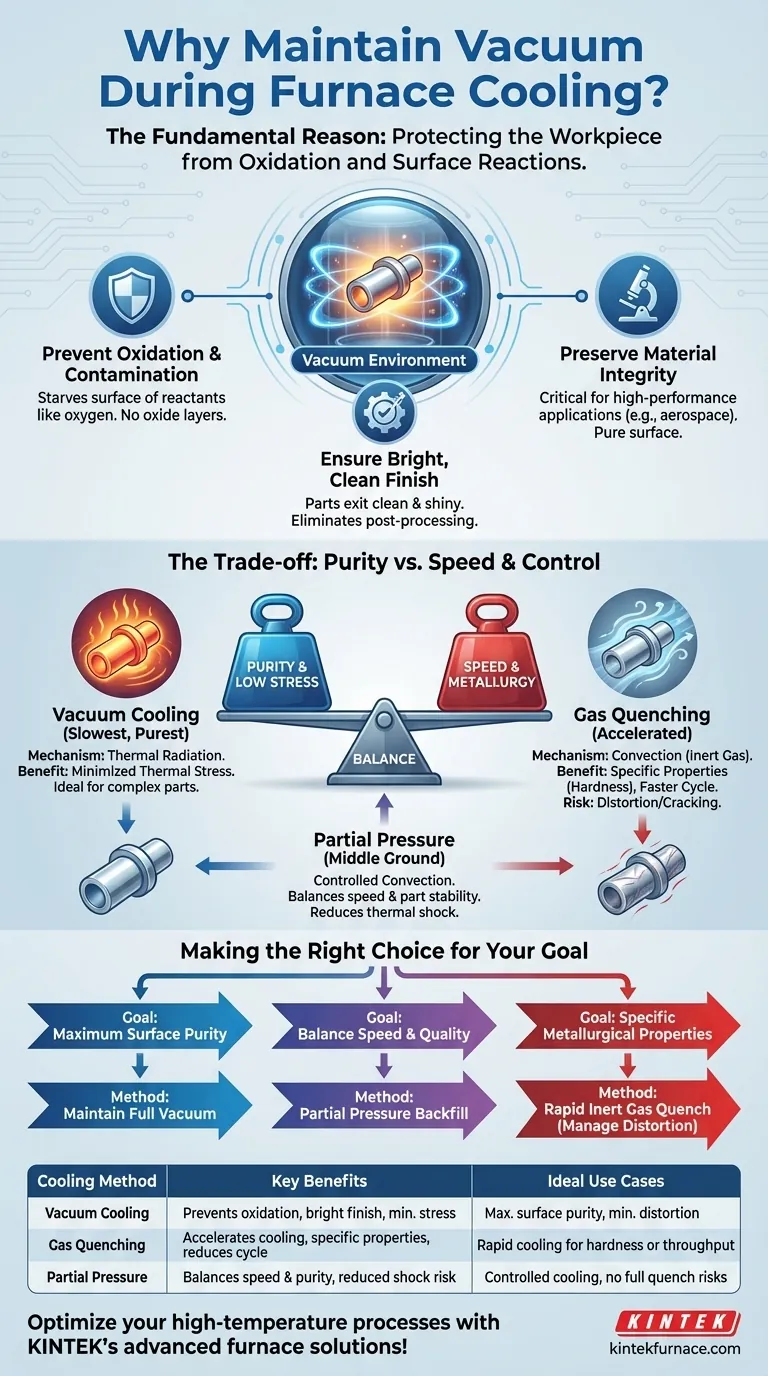
The fundamental reason a vacuum is maintained during cooling in a furnace is to protect the workpiece. At elevated temperatures, metals are highly reactive, and maintaining a vacuum environment prevents oxidation and other surface reactions, ensuring the final part emerges with a clean, bright finish and its intended material properties intact.
The choice to maintain a full vacuum or introduce a gas during cooling is a critical engineering decision. It represents a direct trade-off between achieving maximum surface purity (via vacuum) and controlling the cooling rate to manage cycle time and metallurgical structure (via gas).

The Primary Goal: Protecting the Workpiece
At its core, a vacuum furnace creates a controlled, inert environment. This control is just as critical during the cooling phase as it is during heating.
Preventing Oxidation and Contamination
Even after the heat source is turned off, the workpiece remains hot enough to react instantly with active gases like oxygen or water vapor. Maintaining a vacuum starves the surface of these reactants, preventing the formation of oxides and other undesirable surface layers.
Ensuring a Bright, Clean Finish
This prevention of surface reactions results in a "bright" finish. Parts exit the furnace clean and shiny, often eliminating the need for secondary post-processing steps like acid cleaning, sandblasting, or polishing, which saves both time and cost.
Preserving Material Integrity
For many high-performance applications in aerospace, medical, or electronics, surface integrity is a performance characteristic. A microscopic oxide layer can impede electrical conductivity, alter brazing flow, or create initiation points for fatigue cracks. A vacuum ensures the surface is metallurgically pure.
Controlling the Cooling Rate
While a vacuum is ideal for surface protection, it is not an effective medium for heat transfer. This leads to different strategies for controlling the cooling rate.
Vacuum Cooling: The Slowest, Purest Method
Cooling in a hard vacuum relies almost exclusively on thermal radiation. This is a relatively slow process, as there is no gas to help carry heat away from the part through convection. This gentle, slow cool is ideal for minimizing thermal stress.
Gas Quenching: Accelerating the Process
To speed things up, a process called gas quenching (or backfilling) is used. An inert gas, such as high-purity argon or nitrogen, is pumped into the hot zone. This gas enables convection, dramatically accelerating the rate of heat transfer from the workpiece to the furnace's water-cooled walls.
Why Speed Matters
Rapid cooling, or quenching, is often necessary to achieve specific metallurgical properties, such as locking in a particular grain structure or creating hardness in tool steels. It also significantly shortens the overall process cycle time, increasing throughput.
Understanding the Trade-offs: Purity vs. Speed
The decision of how to cool is not arbitrary; it's a calculated compromise based on the goals for the specific part.
The Purity Compromise
Introducing a quench gas, even one of very high purity, represents a slight deviation from a perfect vacuum. While inert gases don't typically react with the metal, it is a less pure environment than a deep vacuum.
The Risk of Thermal Shock and Distortion
Rapid cooling induced by a gas quench introduces significant thermal stress into the workpiece. For parts with complex geometries, thin walls, or varying cross-sections, this can lead to warping, distortion, or even cracking. A slow, radiative cool in a vacuum minimizes these risks.
Finding the Middle Ground: Partial Pressure
Advanced processes can use a "partial pressure" of inert gas. This introduces just enough gas to speed up cooling through limited convection but not so much that it causes the thermal shock of a full gas quench. This offers a controllable balance between speed and part stability.
Making the Right Choice for Your Goal
Your cooling strategy should be dictated by the final requirements of your component.
- If your primary focus is maximum surface purity and a bright finish: Maintain a full vacuum during cooling, accepting the longer cycle time for a stress-free, uncontaminated part.
- If your primary focus is achieving specific metallurgical properties (like hardness): Use a rapid inert gas quench, but carefully engineer the process to manage the risk of distortion.
- If your primary focus is balancing production speed with part quality: Use a controlled partial pressure backfill to accelerate cooling without inducing excessive thermal shock.
Ultimately, the cooling method is a critical control point used to engineer the final performance and quality of the component.
Summary Table:
| Cooling Method | Key Benefits | Ideal Use Cases |
|---|---|---|
| Vacuum Cooling | Prevents oxidation, ensures bright finish, minimizes thermal stress | Applications requiring maximum surface purity and minimal distortion |
| Gas Quenching | Accelerates cooling, achieves specific metallurgical properties, reduces cycle time | Processes needing rapid cooling for hardness or throughput |
| Partial Pressure | Balances speed and purity, reduces risk of thermal shock | Scenarios requiring controlled cooling without full quench risks |
Optimize your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored high-temperature furnace systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise alignment with your unique experimental needs, enhancing efficiency and results. Contact us today to discuss how we can support your specific requirements and elevate your laboratory's performance!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What properties of a metal can be altered through vacuum heat treating? Enhance Strength, Ductility, and Corrosion Resistance
- What is the core function of a vacuum sublimation furnace in the process of recovering magnesium from scrap magnesium alloys?
- How do industrial furnaces and contact voltage regulators facilitate heat transfer performance testing for sodium heat pipes?
- What are the typical operating voltage and heat output ranges for vacuum furnace heating systems? Ensure Safe, High-Power Performance
- How does a vacuum furnace facilitate precise control of tellurium vacancy concentrations in PtTe2 thin films?
- What role does a high vacuum evaporation system play in Sb2Se3 thin film preparation? Ensure High Purity & Performance
- What types of materials and processes are compatible with vacuum furnaces? Achieve Purity and Precision in Heat Treatment
- What are the advantages of vacuum brazing aluminum compared to traditional welding methods? Superior Joint Integrity and Precision



















