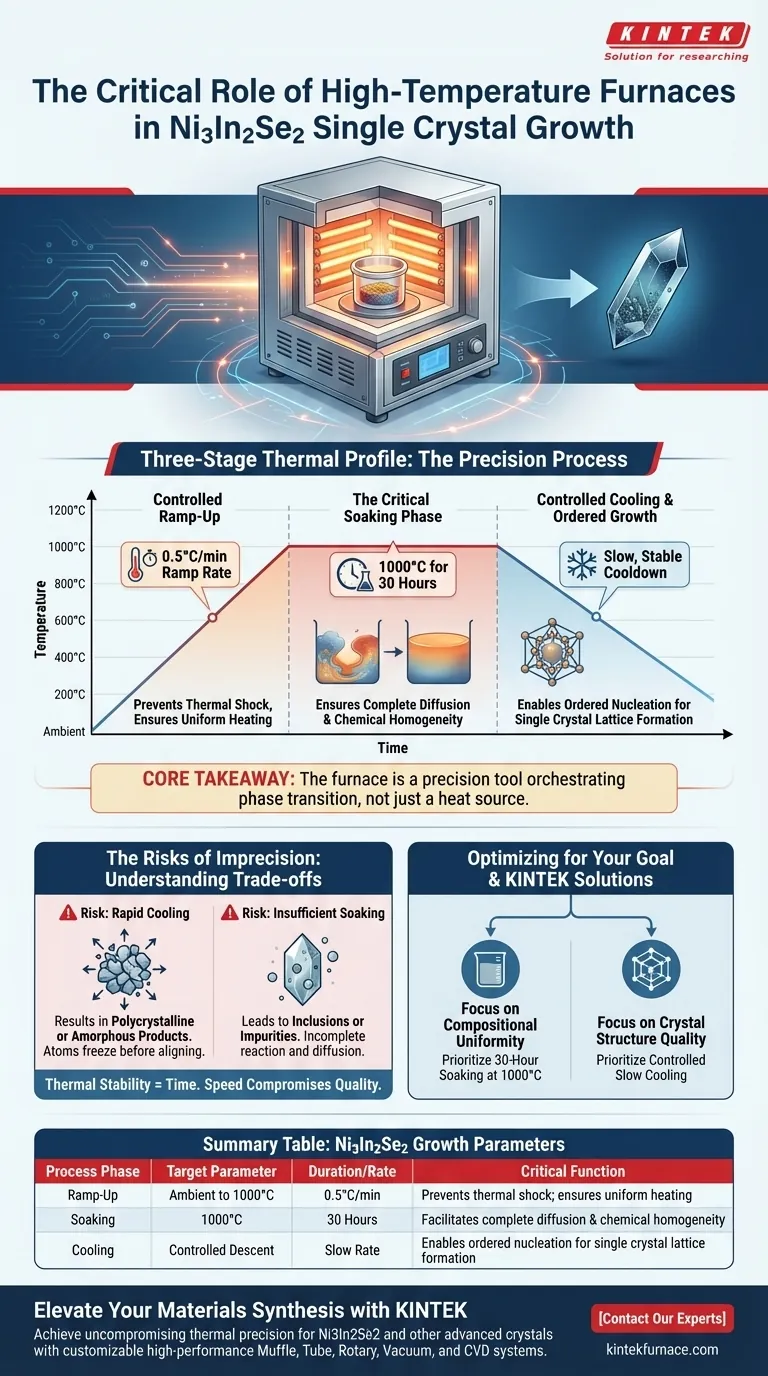
Laboratory high-temperature furnaces serve as the critical reaction environment for the synthesis of Ni3In2Se2 single crystals, specifically by executing a rigorous three-stage thermal profile. To achieve successful growth, these furnaces (typically box furnaces) must ramp temperature at a precise rate of 0.5°C/min up to 1000°C, maintain this heat for 30 hours, and then execute a controlled slow-cooling phase.
Core Takeaway The furnace is not merely a heat source but a precision tool that orchestrates the phase transition of Ni3In2Se2; it ensures complete diffusion through a long high-heat soak and enables ordered nucleation through a highly stable, slow-cooling environment.

The Mechanics of Thermal Control
The growth of Ni3In2Se2 is strictly governed by the thermal field maintained within the furnace. The furnace must manage three distinct phases of the synthesis process to ensure the transition from raw reactants to a single, high-quality crystal.
Controlled Heating and Ramp-Up
The furnace does not simply blast the materials with maximum heat. It utilizes a slow, programmable ramp rate of 0.5°C/min.
This gradual increase allows the reactants to heat uniformly, preventing thermal shock or uneven reaction gradients before the target temperature is reached.
Achieving Homogeneity: The Soaking Phase
Once the furnace reaches 1000°C, it enters a critical "soaking" period that lasts for 30 hours.
This extended duration is essential for the physics of the reaction. It ensures that the raw materials are not just melted, but that complete diffusion occurs throughout the melt.
Without this prolonged high-temperature maintenance, the mixture may remain heterogeneous, leading to inconsistencies in the final crystal structure.
Nucleation and Ordered Growth
The final and perhaps most delicate role of the furnace is the cooling phase. The furnace must provide a stable environment during a controlled, slow cooldown.
This stability allows for the "ordered growth" of the crystal. By lowering the temperature slowly, the furnace prevents rapid solidification, giving the atomic structure time to arrange itself into a single crystal lattice rather than a disordered solid.
Understanding the Trade-offs
While high-temperature box furnaces are effective for this specific growth method, it is vital to understand the operational risks involved in the thermal profile.
The Risk of Rapid Cooling
If the furnace fails to maintain the slow-cooling profile, the environment creates a state of high supersaturation too quickly.
This typically results in polycrystalline or amorphous products rather than a single crystal. The atoms are forced to freeze in place before they can align into the correct lattice structure.
Insufficient Soaking Time
Cutting the 30-hour maintenance period short is a common error.
If the furnace does not hold the 1000°C temperature long enough, the raw materials may not fully react or diffuse. This leads to inclusions or chemical impurities within the crystal matrix.
Thermal Stability vs. Speed
The process is inherently slow. The trade-off for high-quality Ni3In2Se2 growth is time; attempting to accelerate the 0.5°C/min ramp or the 30-hour soak will almost invariably compromise the structural integrity of the crystal.
Making the Right Choice for Your Goal
To replicate the growth of Ni3In2Se2 successfully, you must program your furnace based on the specific outcome you are prioritizing.
- If your primary focus is Compositional Uniformity: Prioritize the 30-hour soaking period at 1000°C to guarantee complete melting and diffusion of the reactants.
- If your primary focus is Crystal Structure Quality: Focus on the controlled slow cooling phase to ensure stable nucleation and prevent polycrystalline formation.
Precision in the thermal profile is the difference between a high-quality single crystal and a failed batch of amorphous material.
Summary Table:
| Process Phase | Target Parameter | Duration/Rate | Critical Function |
|---|---|---|---|
| Ramp-Up | Ambient to 1000°C | 0.5°C/min | Prevents thermal shock; ensures uniform heating |
| Soaking | 1000°C | 30 Hours | Facilitates complete diffusion and chemical homogeneity |
| Cooling | Controlled Descent | Slow Rate | Enables ordered nucleation for single crystal lattice formation |
Elevate Your Materials Synthesis with KINTEK
Achieve the uncompromising thermal precision required for Ni3In2Se2 growth and other advanced crystal synthesis. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your specific laboratory requirements. Whether you need stable 1000°C soaking or ultra-precise ramp rates, our high-temp furnaces provide the stability your research demands.
Ready to optimize your thermal profiles? Contact our experts today to find the perfect furnace solution for your lab!
Visual Guide
References
- Yi Zhou. The Preparation and Physical Properties Study of the Kagome Lattice Semimetal Ni3In2Se2. DOI: 10.47297/taposatwsp2633-456926.20250604
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is vacuum sintering? Achieve Maximum Purity and Density for Advanced Materials
- What are the advantages of vacuum carburizing over conventional atmosphere-carburizing methods? Boost Quality and Efficiency in Case Hardening
- Can horizontal vacuum furnaces be customized for specific needs? Tailor Your Thermal Process for Optimal Results
- What are the advantages of vacuum heat treatment regarding surface quality? Achieve Pristine, Oxidation-Free Surfaces
- What is a vacuum sintering furnace? Achieve High-Purity, Dense Materials
- What happens during the heating phase of a vacuum furnace? Master Radiative Heat and Outgassing Control
- What are the advantages of a mesh belt brazing furnace vs vacuum? Optimize High-Volume Stainless Steel Production
- What technical requirements must a furnace meet for Inconel 718 hardening? Master Precision Aging & Cooling



















