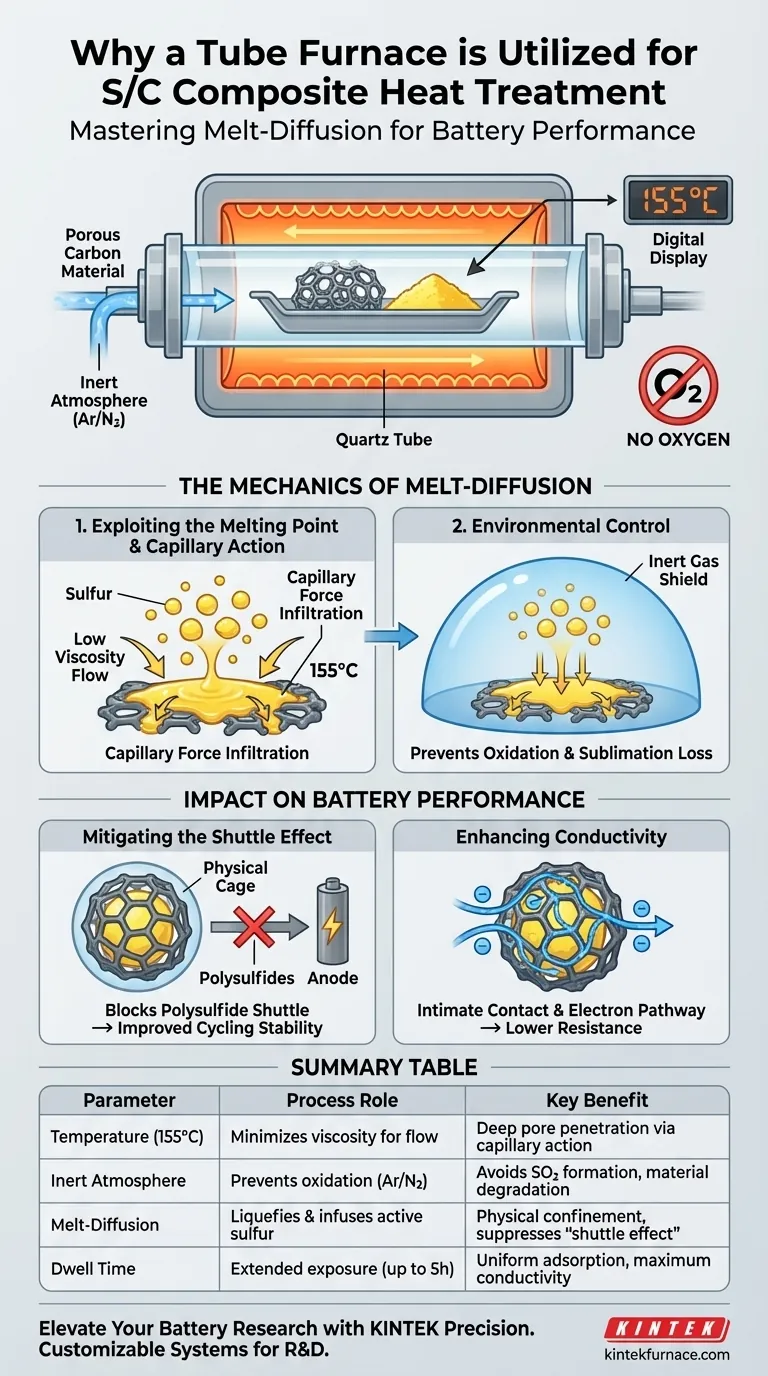
The primary reason for utilizing a tube furnace in the preparation of sulfur/carbon (S/C) composite cathode materials is to execute a precise process known as melt-diffusion under a controlled, inert atmosphere.
By maintaining a constant temperature of approximately 155°C, the furnace liquefies the sulfur, allowing it to penetrate and fill the microscopic pores of the carbon host via capillary action. This technique physically confines the sulfur, which is critical for stabilizing the material and optimizing battery performance.
Core Insight: The tube furnace is not merely a heating element; it is a containment vessel that leverages capillary forces to lock active sulfur inside a conductive carbon skeleton. This physical confinement is the single most effective method for suppressing the parasitic "shuttle effect" that degrades battery life.

The Mechanics of Melt-Diffusion
Exploiting the Melting Point
Sulfur has a melting point near 115°C, but the heat treatment is typically conducted at 155°C.
At this elevated temperature, sulfur achieves the lowest viscosity, allowing it to flow freely. The tube furnace maintains this specific thermal window for an extended period (often up to 5 hours), ensuring the sulfur has ample time to transition into a liquid state suitable for infiltration.
Driving Capillary Action
Once molten, the sulfur does not simply sit on the surface of the carbon.
Due to the low viscosity achieved at 155°C, capillary forces draw the liquid sulfur deep into the carbon substrate. It permeates the highly developed micro-porous and meso-porous structures, effectively impregnating the carbon skeleton with active material.
The Role of Environmental Control
Preventing Oxidation
A tube furnace allows for the introduction of an inert atmosphere, such as argon or nitrogen.
Processing sulfur requires the absolute exclusion of oxygen. If heated in air, sulfur would react to form sulfur dioxide (SO2), degrading the active material and creating toxic byproducts. The sealed environment of the tube furnace prevents this chemical degradation.
Sublimation and Adsorption
Beyond simple melting, the controlled environment allows for the exploitation of sulfur's sublimation properties.
As sulfur sublimates and diffuses, it adsorbs onto the internal surfaces of the porous carbon. This ensures a uniform distribution of active material throughout the composite, rather than just a superficial coating.
Impact on Battery Performance
Mitigating the Shuttle Effect
The primary failure mechanism in lithium-sulfur and magnesium-sulfur batteries is the shuttle effect, where polysulfides dissolve into the electrolyte.
By using the tube furnace to drive sulfur deep into the pores, the carbon structure acts as a physical cage. This confinement restricts the movement of polysulfides, preventing them from shuttling to the anode and significantly improving cycling stability.
Enhancing Conductivity
Sulfur is naturally insulating, which hampers electron flow.
The melt-diffusion process ensures intimately close contact between the insulating sulfur and the conductive carbon network. This creates a robust pathway for electrons, reducing internal resistance and improving the battery's overall electronic conductivity.
Understanding the Trade-offs
Risk of Surface Accumulation
While the goal is pore infiltration, improper execution can lead to surface sulfur accumulation.
If the temperature fluctuates or the heating time is insufficient, sulfur may re-solidify on the exterior of the carbon particles rather than inside the pores. This blocks ion transport channels and renders the encapsulation ineffective.
Volume Expansion Management
Sulfur expands significantly during the discharge cycle.
The tube furnace process relies on the carbon host having enough internal void space to accommodate this expansion. If the pores are over-filled during the melt-diffusion process, the carbon structure may fracture during battery operation, leading to a loss of electrical contact.
Making the Right Choice for Your Goal
When designing a heat treatment protocol for S/C composites, align your parameters with your specific performance targets:
- If your primary focus is Cycle Life: Prioritize longer dwell times at 155°C to ensure maximum pore penetration and physical confinement, minimizing the shuttle effect.
- If your primary focus is Energy Density: Focus on optimizing the sulfur-to-carbon ratio before heating, ensuring you fill the pores completely without leaving excess insulating sulfur on the surface.
The effectiveness of your S/C composite is determined not just by the materials used, but by the precision of the thermal environment that binds them together.
Summary Table:
| Parameter | Process Role | Key Benefit |
|---|---|---|
| Temperature (155°C) | Minimizes sulfur viscosity for flow | Enables deep pore penetration via capillary action |
| Inert Atmosphere | Prevents oxidation (Ar/N2 flow) | Avoids toxic SO2 formation and material degradation |
| Melt-Diffusion | Liquefies and infuses active sulfur | Physical confinement to suppress the 'shuttle effect' |
| Dwell Time | Extended thermal exposure (up to 5h) | Ensures uniform adsorption and maximum conductivity |
Elevate Your Battery Research with KINTEK Precision
High-performance sulfur/carbon composites require more than just heat—they require the absolute thermal precision and atmosphere control that only a specialized laboratory furnace can provide. KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet the rigorous demands of your R&D projects.
Backed by expert manufacturing and deep material science insights, our furnaces ensure the stable, inert environments necessary to eliminate the shuttle effect and maximize cycle life in next-generation battery materials.
Ready to optimize your melt-diffusion process? Contact us today to find your custom heating solution.
Visual Guide
References
- Andrijana Marojević, Jan Bitenc. Influence of Salt Concentration on the Electrochemical Performance of Magnesium Hexafluoroisopropoxy Aluminate Electrolyte. DOI: 10.1002/batt.202500497
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the key features of tube furnaces? Unlock Precision in High-Temperature Processing
- Why is the encapsulation of raw materials in a vacuum-sealed quartz tube necessary for crystal growth? Key to Purity
- What are the primary applications of high temperature tube furnaces? Unlock Precise Heat Control for Materials Science
- What technical conditions does a Tube Furnace provide for silicon nanowire oxidation? Master Nano-Engineering
- What is the role of a high-temperature tube furnace in the preparation of TiO2-alpha-Ga2O3 heterostructures?
- What is a quartz tube furnace and what is its primary use? Essential for Controlled High-Temp Processing
- What core task does a tubular vacuum sintering furnace perform? Optimizing Confined Carbon Chain Synthesis
- Why use a graphite box in tube furnaces for Sb2Se3 annealing? Achieve Precise Crystal Growth & Vapor Control



















