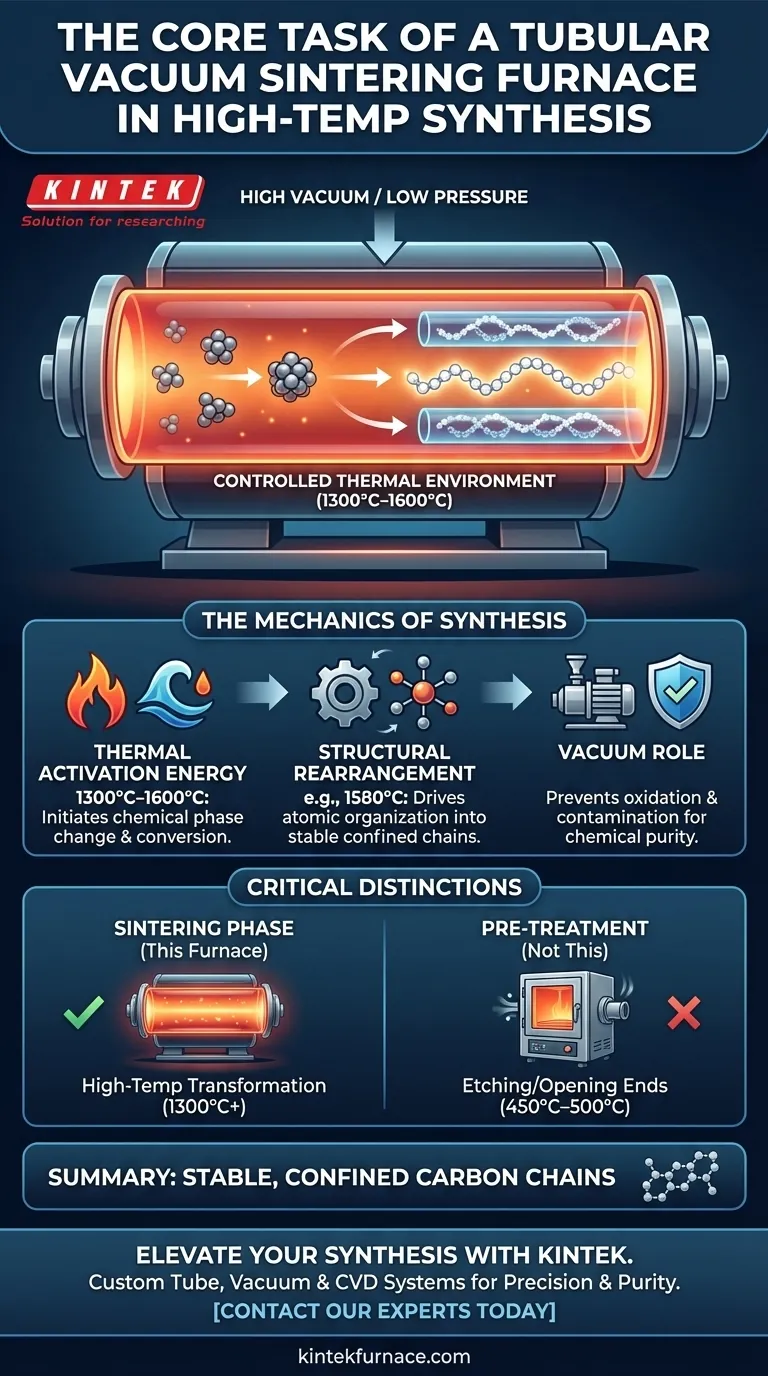
The primary function of a tubular vacuum sintering furnace is to generate a strictly controlled thermal environment—typically between 1300°C and 1600°C—under extremely low pressure. This apparatus supplies the precise activation energy required to drive the structural rearrangement of carbon precursors into stable, confined carbon chains.
By maintaining a high-temperature, low-pressure atmosphere, the furnace facilitates the conversion of precursors without chemical interference. This process is essential for maximizing growth efficiency and ensuring the structural stability of the final nanomaterial.

The Mechanics of High-Temperature Synthesis
Providing Thermal Activation Energy
The central task of the furnace is to supply the thermal energy needed to initiate a chemical phase change. During synthesis, the temperature is maintained between 1300°C and 1600°C.
This intense heat provides the activation energy necessary for the carbon precursors—which are confined within nanotubes—to undergo conversion. Without this specific thermal threshold, the precursors would remain inert and fail to form the desired chain structures.
Facilitating Structural Rearrangement
Beyond simple heating, the furnace drives the structural rearrangement of the carbon atoms. The process is not merely about melting or fusing, but about organizing atoms into a specific, stable configuration inside the nanotube.
Precise temperature regulation is critical here. For example, operating at specific set points like 1580°C has been shown to maximize growth efficiency and ensure the resulting product maintains structural integrity.
The Role of the Vacuum Environment
The "vacuum" component of the furnace is just as critical as the heat. By operating at extremely low pressure, the furnace creates a reaction environment that is nearly neutral.
This prevents the material from reacting with its surroundings. A high degree of vacuum mitigates the risk of oxidation or contamination, ensuring that the synthesis focuses solely on the internal rearrangement of the carbon chains.
Critical Process Distinctions
Sintering vs. Pre-treatment
It is vital to distinguish the high-temperature sintering phase from the pre-treatment phase. Before sintering, an air oxidation furnace is often used at much lower temperatures (450°C–500°C) to etch open the ends of nanotubes.
The tubular vacuum sintering furnace is not used for this opening process. Its role is strictly the high-temperature transformation (1300°C+) that occurs after the precursors have entered the open nanotubes.
Temperature Uniformity and Purity
While the primary reference highlights temperature range, the uniformity of that temperature is a key trade-off in equipment selection. High-temperature tube furnaces are designed to offer precisely controlled temperature zones.
Lack of uniformity can lead to incomplete crystal structures. To ensure phase purity, the thermal energy must be applied evenly across the entire length of the reaction zone.
Making the Right Choice for Your Goal
When configuring your synthesis protocol, your equipment settings must align with your specific objectives.
- If your primary focus is Growth Efficiency: Target precise temperature regulation points, such as 1580°C, to maximize the conversion rate of precursors.
- If your primary focus is Phase Purity: prioritize a furnace with exceptional temperature uniformity and high-vacuum capabilities to eliminate environmental interference.
- If your primary focus is Precursor Filling: Do not use the sintering furnace; utilize an air oxidation furnace at 450°C–500°C to open nanotube caps first.
Success in synthesizing confined carbon chains relies on isolating the thermal conversion process from atmospheric variables.
Summary Table:
| Feature | Specification/Requirement | Role in Synthesis |
|---|---|---|
| Temperature Range | 1300°C – 1600°C | Provides activation energy for atomic rearrangement |
| Optimal Set Point | 1580°C | Maximizes growth efficiency and structural stability |
| Atmosphere | High Vacuum / Low Pressure | Prevents oxidation and ensures chemical purity |
| Process Focus | Structural Transformation | Converts precursors into stable confined carbon chains |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is non-negotiable when working at the 1600°C threshold. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to deliver the thermal uniformity and high-vacuum integrity required for advanced carbon research.
Backed by expert R&D and manufacturing, our furnaces are fully customizable to meet your unique laboratory needs. Whether you are focusing on growth efficiency or phase purity, KINTEK high-temperature solutions ensure your materials maintain their structural integrity every time.
Ready to optimize your synthesis protocol?
→ Contact Our Technical Experts Today
Visual Guide
References
- Clara Freytag, Thomas Pichler. Systematic Optimization of the Synthesis of Confined Carbyne. DOI: 10.1002/smtd.202500075
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What types of heating elements are used in a 70mm tube furnace? Optimize Your High-Temp Processes
- How do high-temperature tube furnaces optimize the performance of ceramic materials during post-sintering annealing?
- How are vacuum tube furnaces utilized in the metallurgical industry? Enhance Metal Purity and Performance
- What are the main operational considerations when using a lab tube furnace? Ensure Precision and Safety in Your Experiments
- How do split tube furnaces provide access to the chamber? Unlock Easy Sample Handling for Your Lab
- What is the significance of using a high-temperature tube furnace with observation windows? Real-Time Wettability Analysis
- What role does a tube furnace play in g-C3N4 thin film preparation? Optimize Your Hot-Wall CVD Synthesis
- What conditions does a tube furnace provide for aluminum ash-based ceramsite roasting? Master Precision Sintering



















