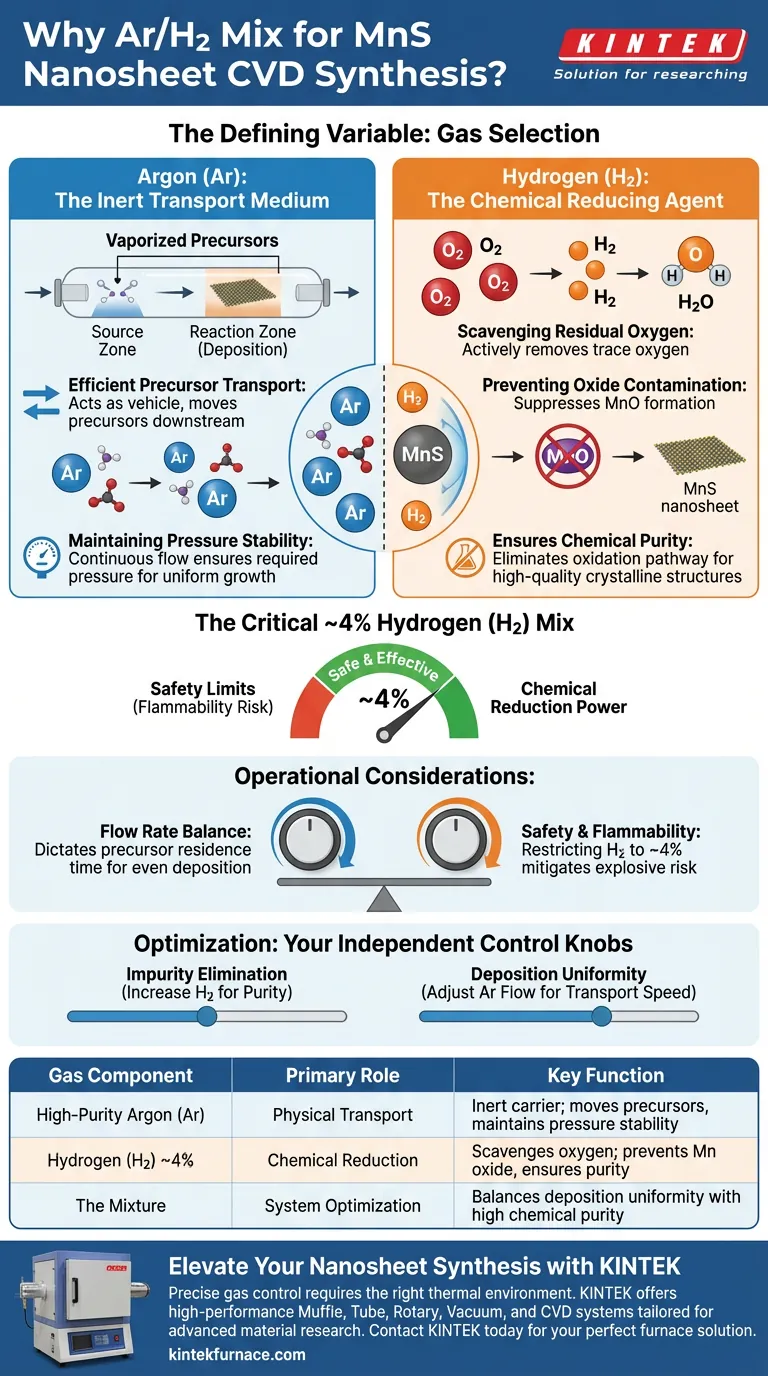
The selection of carrier gas is a defining variable in Chemical Vapor Deposition (CVD). To synthesize Manganese Sulfide (MnS) nanosheets, a mixture of high-purity Argon (Ar) and Hydrogen (H2) is used to satisfy both physical and chemical requirements. Argon serves as the inert transport medium for precursors, while Hydrogen acts as a reducing agent to eliminate oxygen and prevent the material from degrading into oxides.
Core Insight: While Argon provides the physical mass transport necessary to move vaporized precursors through the system, it is the addition of approximately 4% Hydrogen that creates a critical reducing atmosphere. This chemical intervention neutralizes residual oxygen, preventing the formation of manganese oxides and guaranteeing the high purity of the final MnS nanosheets.
The Physical Role of Argon
In the CVD process, Argon acts as the mechanical backbone of the system. It is chosen for its chemical inertness, meaning it will not participate in the reaction itself.
Efficient Precursor Transport
The primary function of high-purity Argon is to act as a vehicle for the vaporized precursors. It sweeps these materials from the source zone and carries them downstream to the reaction zone where deposition occurs.
Maintaining Pressure Stability
A stable pressure environment is essential for uniform nanosheet growth. The continuous flow of Argon helps maintain the required internal pressure within the CVD tube throughout the synthesis duration.
The Chemical Role of Hydrogen
While Argon handles transport, Hydrogen addresses the specific chemical vulnerability of Manganese. Manganese is prone to oxidation, which requires active mitigation.
Scavenging Residual Oxygen
Even in controlled environments, trace amounts of oxygen may persist. The addition of Hydrogen (H2) introduces a reducing atmosphere that actively reacts with and removes this residual oxygen.
Preventing Oxide Contamination
Without Hydrogen, oxygen would react with the precursors to form manganese oxides rather than the desired sulfide. The reducing environment effectively suppresses this side reaction.
Ensuring Chemical Purity
By eliminating the pathway for oxidation, the process ensures that the synthesized nanosheets are composed of pure Manganese Sulfide (MnS). This leads to high-quality, chemically accurate crystalline structures.
Operational Considerations
While this gas mixture is effective, it introduces specific operational variables that must be managed to ensure safety and efficiency.
Safety Limits and Flammability
Hydrogen is highly flammable. By restricting the concentration to approximately 4%, the mixture remains effective for reduction while mitigating the explosive risks associated with higher Hydrogen concentrations.
Flow Rate Balance
The total flow rate of the mixture dictates the residence time of the precursors. If the flow is too fast, precursors may exit the tube before depositing; if too slow, the deposition may be uneven.
Optimizing Your CVD Parameters
To achieve the best results, you must view these gases as independent control knobs for physical transport and chemical purity.
- If your primary focus is eliminating impurities: Ensure your Hydrogen concentration is sufficient (around 4%) to fully neutralize any oxygen leaks or residuals in the system.
- If your primary focus is deposition uniformity: Adjust the Argon flow rate to control the speed of precursor transport without altering the chemical reducing potential.
Mastering the ratio and flow of this Argon-Hydrogen mixture is the key to transitioning from rough, oxidized samples to pristine MnS nanosheets.
Summary Table:
| Gas Component | Primary Role | Key Function in MnS Synthesis |
|---|---|---|
| High-Purity Argon (Ar) | Physical Transport | Inert carrier; moves precursors and maintains pressure stability. |
| Hydrogen (H2) ~4% | Chemical Reduction | Scavenges residual oxygen; prevents manganese oxide formation. |
| The Mixture | System Optimization | Balances deposition uniformity with high chemical purity. |
Elevate Your Nanosheet Synthesis with KINTEK
Precise gas control is only half the battle—the right thermal environment is the other. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for advanced material research. Whether you are synthesizing MnS nanosheets or exploring new 2D materials, our customizable lab high-temp furnaces provide the stability and control your unique needs demand.
Ready to optimize your CVD process? Contact KINTEK today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Chaojie Xie, Yu Zhao. A Broadband Photodetector Based on Non-Layered MnS/WSe2 Type-I Heterojunctions with Ultrahigh Photoresponsivity and Fast Photoresponse. DOI: 10.3390/ma17071590
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the structural advantages of a customized AP-SCVD system? High-Throughput WO3 Thin Film Production
- What is the function of a horizontal hot-wall quartz tube CVD system? Expert Insights on Superlattice Fabrication
- What other specialized fields utilize CVD furnaces? Explore Aerospace, Optics, Energy & Material Science
- What role does a Chemical Vapor Deposition (CVD) system play in CsPbBr3 film growth? Master Single-Crystal Precision.
- What are the advantages of using quartz tubes in CVD furnaces? Ensure Purity and Stability for Thin Film Deposition
- What are intermetallic compounds, and how are they used in CVD? Unlock Advanced Thin Film Solutions
- What role does molten Tin (Sn) play in B-CVD growth of graphene? Engineering High-Performance Wrinkled Structures
- What are the disadvantages of CVD coating? High Heat, Toxic By-Products, and Cost Challenges



















