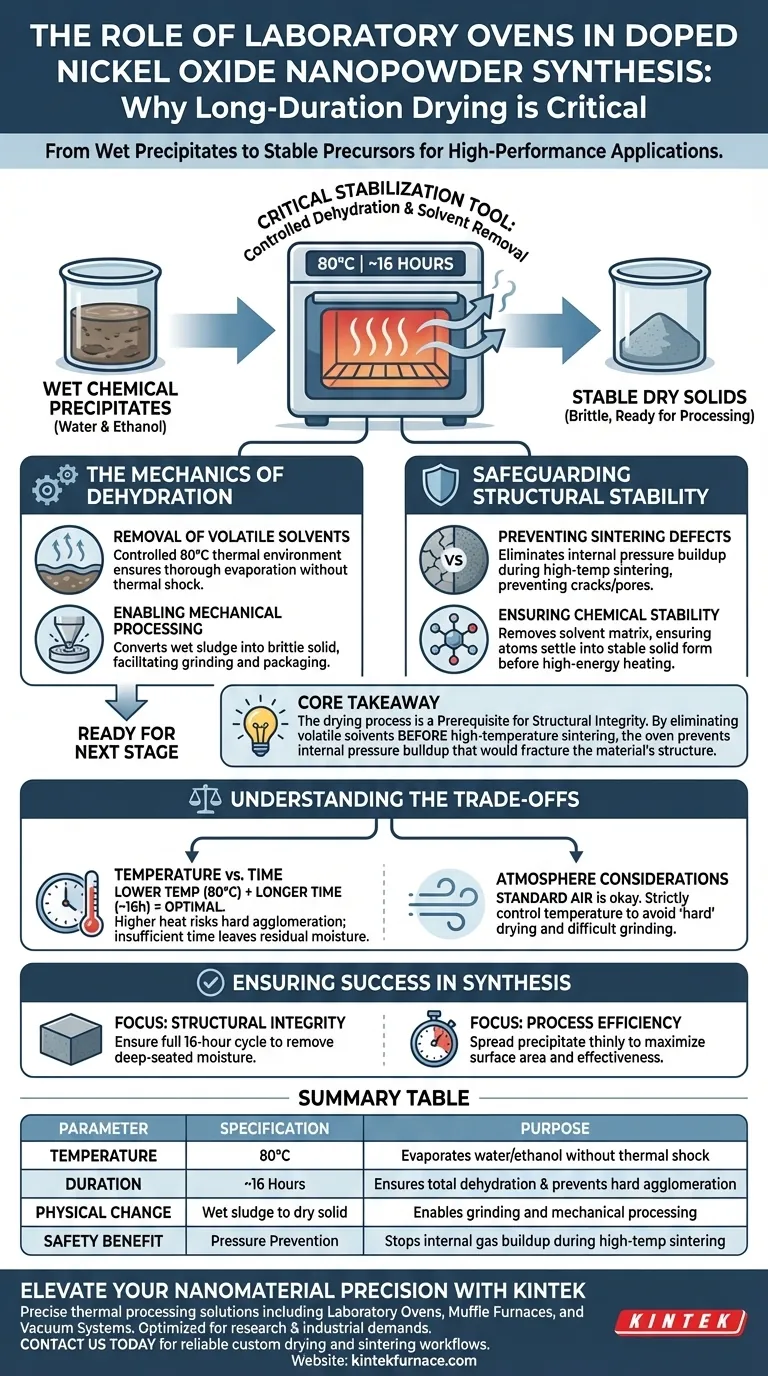
The laboratory oven acts as a critical stabilization tool during the synthesis of doped Nickel Oxide nanopowders. It is specifically required to dehydrate washed precipitates—typically maintaining a temperature of 80°C for approximately 16 hours. This long-duration exposure effectively removes surface moisture and residual ethanol solvents, converting wet chemical raw materials into stable dry solids.
Core Takeaway The drying process is not merely about evaporation; it is a prerequisite for structural integrity. By eliminating volatile solvents before the high-temperature sintering stage, the oven prevents internal pressure buildup that would otherwise fracture the material's structure.

The Mechanics of Dehydration
The synthesis of nanopowders often begins with wet chemical precipitation. The laboratory oven bridges the gap between this wet phase and the final solid state.
Removal of Volatile Solvents
After the initial washing steps, the precipitates retain significant amounts of water and ethanol. The oven provides a controlled thermal environment to drive off these volatiles. Operating at 80°C ensures thorough evaporation without subjecting the material to thermal shock.
Enabling Mechanical Processing
Wet precipitates are sludge-like and impossible to process mechanically. Long-duration drying converts this sludge into a dry, brittle solid. This physical transformation is necessary to facilitate subsequent grinding and packaging steps.
Safeguarding Structural Stability
The most critical function of the laboratory oven is protecting the nanomaterial during future processing steps.
Preventing Sintering Defects
Following drying, these materials often undergo high-temperature sintering. If excess water or solvent remains within the material, the intense heat of sintering would cause rapid vaporization. This rapid expansion of gas would disrupt the material structure, leading to cracks or pores that compromise the final quality.
Ensuring Chemical Stability
The drying phase ensures the precipitate is chemically stable before it enters high-energy environments. By removing the solvent matrix, the oven ensures that the atoms settle into a stable solid form. This reduces the risk of structural collapse or unintended phase changes during later heating stages.
Understanding the Trade-offs
While the laboratory oven is essential, the parameters of its use involve specific trade-offs that affect the final product.
Temperature vs. Time
A lower temperature (80°C) is used deliberately, necessitating a longer duration (16 hours). Rushing this process with higher heat could lead to hard agglomeration—where particles fuse together tightly. Conversely, insufficient time leaves residual moisture, rendering the material unsuitable for sintering.
Atmosphere Considerations
Standard ovens operate in air, which is generally acceptable for Nickel Oxide. However, strictly controlling the temperature is vital. Excessive heat during drying can lead to "hard" drying, making the subsequent grinding process difficult and potentially altering the particle size distribution.
Ensuring Success in Synthesis
To maximize the quality of your doped Nickel Oxide nanopowders, align your drying strategy with your specific processing goals.
- If your primary focus is Structural Integrity: Ensure the full 16-hour cycle is completed to remove all deep-seated moisture that could cause cracking during sintering.
- If your primary focus is Process Efficiency: Verify that the precipitate is spread thinly to maximize surface area, ensuring the 16-hour duration is fully effective rather than needing extended time.
Proper drying transforms a volatile intermediate into a robust precursor ready for high-performance applications.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Temperature | 80°C | Evaporates water/ethanol without thermal shock |
| Duration | ~16 Hours | Ensures total dehydration & prevents hard agglomeration |
| Physical Change | Wet sludge to dry solid | Enables grinding and mechanical processing |
| Safety Benefit | Pressure Prevention | Stops internal gas buildup during high-temp sintering |
Elevate Your Nanomaterial Precision with KINTEK
Precise thermal processing is the backbone of high-performance nanopowder synthesis. At KINTEK, we understand that every degree and hour counts toward achieving structural integrity. Backed by expert R&D and manufacturing, we offer high-precision Laboratory Ovens, Muffle Furnaces, and Vacuum systems tailored for the rigorous demands of researchers and industrial manufacturers.
Whether you need custom dimensions or advanced programmable controls for doped Nickel Oxide synthesis, KINTEK provides the reliability your lab requires. Contact us today to optimize your drying and sintering workflows!
Visual Guide
References
- Farzaneh Asaldoust, Maryam Taleb-Abbasi. Structural, magnetic, and optical characteristics of undoped and chromium, iron, cobalt, copper, and zinc doped nickel oxide nanopowders. DOI: 10.1038/s41598-025-85239-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the key design features of box furnaces? Discover High-Performance, Safe Thermal Processing
- What role does a Muffle Furnace play in high-belite cement clinker? Optimize Sintering with Precision Control
- What is the purpose of using a high-temperature muffle furnace for post-annealing? Enhance Metal Oxide Performance
- How is an industrial-grade ashing furnace utilized in 3D-printed bioactive glass? Master Debinding & Sintering
- Why is an industrial muffle furnace required for Zirconia supports? Engineering High-Performance Catalyst Platforms
- How should metal materials with grease be handled in a muffle furnace? Prevent Damage and Extend Furnace Life
- What role does a high-temperature muffle furnace play in KNN-based ceramic powder pre-sintering? Key Synthesis Insights
- What is the primary use of a muffle furnace in the assembly of side-heated resistive gas sensors? Expert Annealing Guide



















