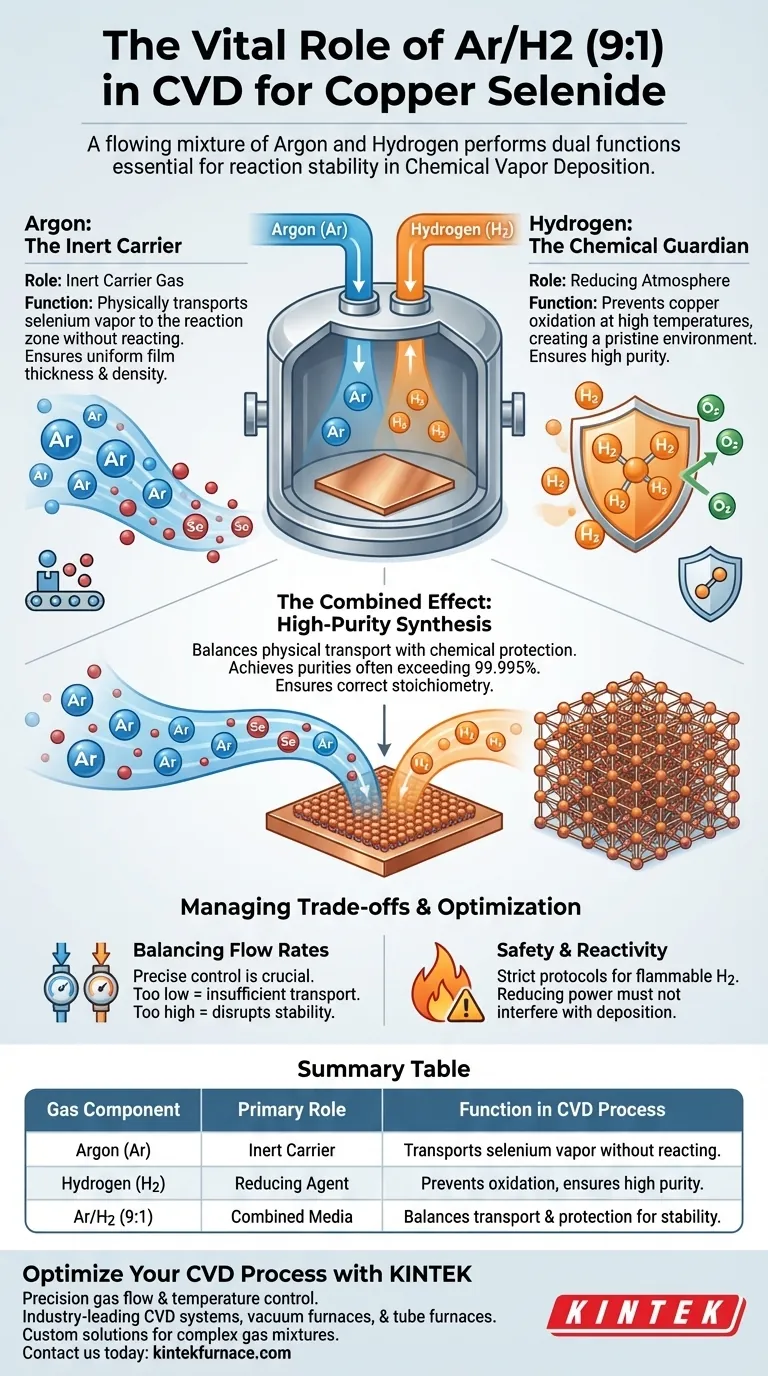
The flowing mixture of Argon and Hydrogen (Ar/H2) performs two distinct but complementary functions essential for reaction stability. Argon acts as the physical transport mechanism for the reactants, while Hydrogen creates a chemical shield against impurities. Without this precise combination, the synthesis of pure copper selenide would be compromised by oxidation and inconsistent delivery.
The core challenge in Chemical Vapor Deposition (CVD) is delivering reactants while maintaining a pristine environment. The Ar/H2 mixture addresses this by using an inert carrier to move selenium vapor and a reducing agent to prevent copper oxidation, ensuring the final material meets high-purity standards.

The Mechanics of the Gas Mixture
The 9:1 mixture is not arbitrary; it represents a balance between physical transport and chemical protection. Each component addresses a specific requirement of the CVD process.
Argon: The Inert Carrier
Argon (Ar) serves as the "vehicle" in this process. Its primary role is to act as a carrier gas.
Because Argon is chemically inert, it does not participate in the reaction itself. Instead, it creates a steady flow that transports the selenium vapor from its source to the copper foil substrate.
This steady transport ensures that the selenium is delivered consistently to the reaction zone, which is vital for achieving uniform film thickness and density.
Hydrogen: The Chemical Guardian
Hydrogen (H2) serves as the "shield." Its primary role is to provide a reducing atmosphere.
CVD processes typically require high temperatures to initiate chemical reactions. At these elevated temperatures, the copper foil substrate is highly susceptible to reaction with any residual oxygen, which leads to oxidation.
Hydrogen inhibits this oxidation. By reacting with potential oxidizers, it maintains a pure environment, ensuring the selenium reacts directly with the copper rather than interacting with copper oxides.
The Result: High-Purity Synthesis
The combined effect of these gases directly influences the quality of the final material.
Preventing Contamination
One of the primary advantages of CVD is the ability to produce materials with purity often exceeding 99.995%.
The presence of Hydrogen is critical to maintaining this standard. If the copper foil were to oxidize, impurities and defects would be introduced into the crystal lattice of the copper selenide.
Ensuring Correct Stoichiometry
For copper selenide to form correctly, the reaction must occur between pure copper and selenium vapor.
By stripping away oxygen and preventing the formation of oxides, the gas mixture ensures the chemical reaction follows the intended pathway. This allows the material to conform homogeneously to the substrate and achieve near-theoretical density.
Understanding the Trade-offs
While necessary, using this specific gas mixture requires careful management of process variables.
Balancing Flow Rates
The flow rate of the Ar/H2 mixture must be precisely controlled.
If the flow is too low, the transport of selenium vapor may be insufficient, leading to slow growth rates or uneven coverage. If the flow is too high, it may disrupt the temperature stability of the substrate or blow reactants away before they can deposit.
Safety and Reactivity
Hydrogen is highly flammable. While essential for reducing oxidation, introducing it into a high-temperature furnace requires strict safety protocols to prevent combustion outside the controlled reaction zone.
Additionally, the "reducing" power of hydrogen must be balanced; it is meant to reduce oxides, not to interfere with the primary deposition of the selenide structure.
Making the Right Choice for Your Goal
When optimizing your CVD process for copper selenide, consider how your specific goals influence how you manage this gas mixture.
- If your primary focus is Purity: Prioritize the Hydrogen concentration and ensure the system is leak-proof to maintain a strictly reducing atmosphere that eliminates all oxides.
- If your primary focus is Uniformity: Focus on the Argon flow rate stability to ensure the selenium vapor is transported evenly across the entire surface of the copper foil.
By mastering the dual roles of transport and protection, you ensure the synthesis of high-quality, defect-free copper selenide.
Summary Table:
| Gas Component | Primary Role | Function in CVD Process |
|---|---|---|
| Argon (Ar) | Inert Carrier | Transports selenium vapor to the substrate without reacting. |
| Hydrogen (H2) | Reducing Agent | Prevents copper oxidation and ensures high material purity. |
| Ar/H2 (9:1) | Combined Media | Balances physical transport with chemical protection for stability. |
Optimize Your CVD Process with KINTEK
Precision in gas flow and temperature control is the difference between a failed run and a high-purity synthesis. KINTEK provides industry-leading CVD systems, vacuum furnaces, and tube furnaces designed to handle complex gas mixtures like Ar/H2 with absolute safety and accuracy.
Backed by expert R&D and specialized manufacturing, our equipment is fully customizable to meet your unique laboratory requirements. Whether you are synthesizing copper selenide or developing next-generation thin films, our systems ensure the uniform heating and atmospheric stability you need.
Ready to elevate your material science? Contact us today to find your custom furnace solution!
Visual Guide
References
- Rajesh Rajasekharan, Manikoth M. Shaijumon. Bifunctional Current Collectors for Lean‐Lithium Metal Batteries. DOI: 10.1002/adfm.202502473
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a high-vacuum thermal evaporation system required for gold back electrodes? Ensure Pure, High-Efficiency Contacts
- What is the function of a Liquid Source Chemical Vapor Deposition (LSCVD) system? Precision CNT Synthesis for Composites
- What is the summary of the CVD process? Master Thin Film Deposition for High-Performance Materials
- What are the main synthetic methods for 2D materials? Choose the Right Method for Your Application
- How are hexagonal boron nitride (h-BN) films processed using CVD tube furnaces? Optimize Growth for High-Quality 2D Materials
- Importance of Quartz Boat Positioning in CVD Growth of Beta-Cu2-xSe: Achieve Precise Phase Purity
- What are the main advantages of CVD? Achieve Superior Film Deposition for Your Applications
- Why are high-purity hydrogen and argon necessary for hBN thin film LPCVD? Master Gas Roles for Superior Growth



















