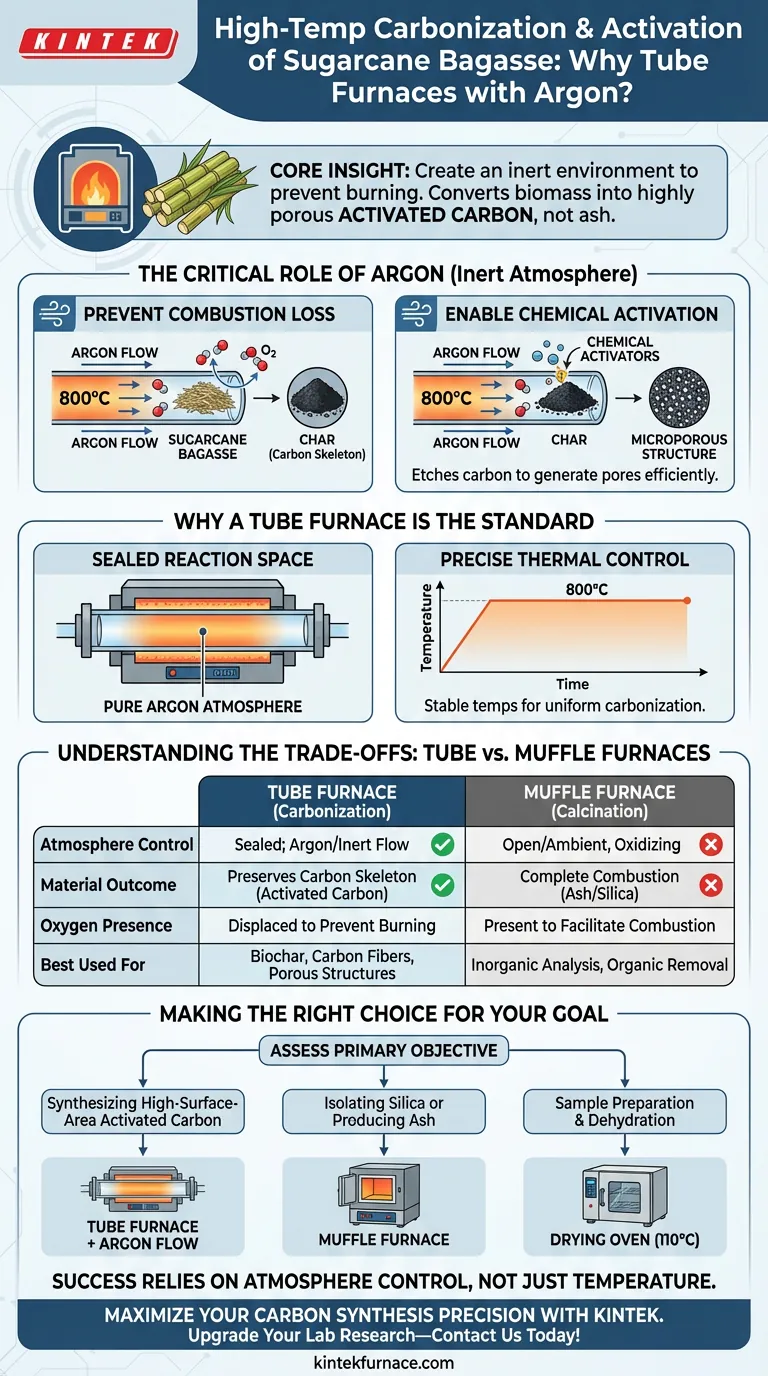
The primary reason for using a tube furnace with argon flow is to create an inert environment that prevents the sugarcane bagasse from burning into ash at high temperatures.
This setup allows for the precise thermal treatment required to convert raw biomass into highly porous activated carbon. By displacing oxygen, the argon ensures that the carbon skeleton remains intact while chemical agents create the desired microporous structure.
Core Insight: High-temperature activation involves a delicate balance: you must heat the material enough to trigger chemical reactions, but prevent the oxidation that destroys carbon. The tube furnace provides the sealed vessel, and argon provides the protective atmosphere, ensuring the process yields a high-surface-area material rather than a pile of ash.

The Critical Role of the Inert Atmosphere
Preventing Combustion Loss
At temperatures such as 800 °C, carbon materials are highly reactive with oxygen. Without a protective gas shield, the carbon in the sugarcane bagasse would simply combust.
Argon acts as an inert barrier, displacing the air within the tube. This prevents the loss of carbon material, ensuring the biomass is converted into char rather than burned away.
Enabling Chemical Activation
The goal of this process is often to create a "microporous" structure—a material filled with tiny holes that increase surface area.
By maintaining an oxygen-free environment, chemical activators (like sodium hydroxide) can react fully with the carbon skeleton. This reaction "etches" the carbon to generate pores efficiently, rather than consuming the material entirely.
Why a Tube Furnace is the Standard
Sealed Reaction Space
Unlike open heating methods, a tube furnace provides a tightly sealed reaction chamber. This confinement is essential for maintaining the purity of the argon atmosphere throughout the process.
Precise Thermal Control
Carbonization requires holding specific temperatures (often around 800 °C) for exact durations. Tube furnaces offer the stability needed to keep these temperatures constant, ensuring uniform carbonization across the entire sample.
Understanding the Trade-offs: Tube vs. Muffle Furnaces
It is crucial to select the right furnace based on your desired end-product. Using the wrong equipment will lead to complete failure of the experiment.
Tube Furnace (Carbonization)
Use this for: Creating activated carbon, biochar, or carbon fibers. Mechanism: The sealed environment limits oxygen. It preserves the carbon structure and allows for the development of porosity and low-density frameworks.
Muffle Furnace (Calcination)
Use this for: Creating ash or isolating silica. Mechanism: Muffle furnaces typically allow for oxygen presence, leading to complete carbon combustion. This is useful if you want to remove all organic matter to study inorganic components like silica, but it destroys the carbon material you are trying to create in this specific context.
Making the Right Choice for Your Goal
To ensure you are using the correct setup for your sugarcane bagasse processing, assess your primary objective:
- If your primary focus is synthesizing high-surface-area activated carbon: Use a tube furnace with argon flow to prevent combustion and enable chemical activators to generate micropores.
- If your primary focus is isolating silica or producing ash: Use a muffle furnace to allow for full combustion (calcination) and the removal of carbon.
- If your primary focus is sample preparation and dehydration: Use a constant temperature drying oven (typically at 110 °C) to remove moisture before high-temperature processing.
The success of converting biomass into advanced carbon materials relies entirely on your ability to control the atmosphere, not just the temperature.
Summary Table:
| Feature | Tube Furnace (Carbonization) | Muffle Furnace (Calcination) |
|---|---|---|
| Atmosphere Control | Sealed; Argon/Inert flow | Open/Ambient; Oxidizing |
| Material Outcome | Preserves carbon skeleton (Activated Carbon) | Complete combustion (Ash/Silica) |
| Oxygen Presence | Displaced to prevent burning | Present to facilitate combustion |
| Best Used For | Biochar, carbon fibers, porous structures | Inorganic analysis, organic removal |
Maximize Your Carbon Synthesis Precision with KINTEK
Don't let your biomass turn to ash—ensure optimal porosity and yield with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory requirements.
Whether you are scaling up carbonization or refining chemical activation, our experts are here to help you select the perfect system.
Upgrade Your Lab Research—Contact Us Today!
Visual Guide
References
- Kyfti Yolanda Siburian, Agung Nugroho. Effect of CoO loading on electrochemical properties of activated carbon from sugarcane bagasse. DOI: 10.5599/jese.2439
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What industries commonly use horizontal furnaces? Unlock High-Temperature Processing for Your Sector
- What are the advantages of individually temperature-controlled zones in multi-zone furnaces? Unlock Precision Thermal Gradients
- Why is uniform heating important in tubular furnaces? Ensure Process Reliability and Predictable Results
- What materials are used for the tube chamber in tubular furnaces? Choose the Right Tube for Your Lab's High-Temp Needs
- What role does a laboratory tube furnace play in the carbonization process of moxa floss? Expert Guide to Biomass Synthesis
- What technical features make a laboratory horizontal tube furnace an ideal reaction device for oil sludge studies?
- Why is a long-term annealing process in a tube furnace essential for Bi-Sb alloy? Achieve Material Homogeneity
- Why is alumina ceramic tubing selected as the liner for a Drop Tube Furnace? Ensure Purity and High-Temp Stability



















