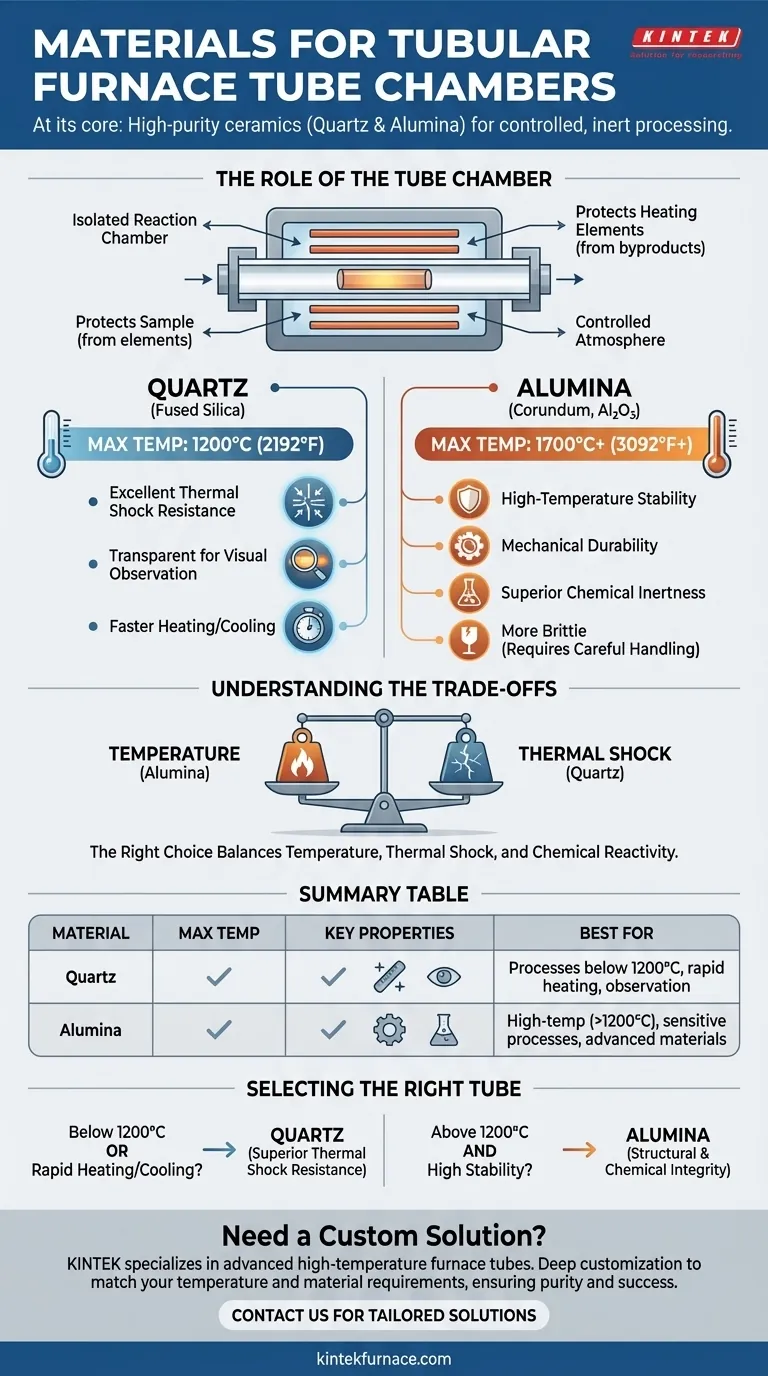
At its core, the tube chamber in a tubular furnace is constructed from high-purity, temperature-resistant ceramics. The two most prevalent materials used for this critical component are quartz (fused silica) and alumina (also known as corundum or aluminum oxide). This choice is dictated by the need to create a chemically inert and thermally stable environment for processing sensitive materials.
The selection of a tube material is not a trivial detail; it is a fundamental engineering decision. The right choice balances the maximum required operating temperature against the material's resistance to thermal shock and chemical reactivity, directly impacting the success and purity of your experiment.

The Role of the Tube Chamber
The tube is the heart of the furnace, serving as an isolated reaction chamber. Its primary function is to contain the sample and any process gases, creating a controlled atmosphere.
It simultaneously protects the sample from contamination by the heating elements and protects the heating elements from any volatile byproducts created during the process. The material integrity of this tube is therefore paramount.
A Breakdown of Key Materials
The choice between quartz and alumina depends entirely on the specific demands of the thermal process. Each material offers a distinct set of properties.
Quartz (Fused Silica)
Quartz is an excellent, versatile material for a wide range of applications. Its key advantage is its outstanding resistance to thermal shock. This allows for faster heating and cooling rates without the risk of the tube cracking.
It is typically suitable for continuous use at temperatures up to 1200°C (2192°F). Because it is transparent, it also allows for direct visual observation of the sample during the process, which can be invaluable in research and development.
Alumina (Corundum, Al₂O₃)
Alumina is the material of choice for very high-temperature applications. Depending on its purity, alumina can be used at temperatures up to 1700°C (3092°F) or even higher.
It is extremely hard, mechanically durable at high temperatures, and possesses excellent chemical inertness. This makes it ideal for processing advanced materials, crystal growth, and other demanding applications where temperature requirements exceed the limits of quartz.
Understanding the Trade-offs
Choosing a material involves navigating a clear set of engineering trade-offs. An incorrect choice can lead to failed experiments, damaged equipment, and contaminated samples.
Temperature vs. Thermal Shock
This is the most critical trade-off. Alumina can withstand significantly higher temperatures, but it is more brittle and susceptible to cracking if heated or cooled too quickly. Quartz excels with rapid temperature changes but has a lower maximum operating temperature.
Chemical Inertness and Purity
Both materials are valued for being highly unreactive, which is essential for maintaining sample integrity. High-purity alumina is often considered superior for processes that are extremely sensitive to contamination, particularly at the highest temperature ranges.
Cost and Handling
Generally, quartz tubes are more cost-effective for applications that fall within their operational temperature range. Alumina tubes, especially those of very high purity, can be more expensive. Their brittleness also requires more careful handling to prevent mechanical fractures.
Selecting the Right Tube for Your Application
Your choice should be guided by the primary goal of your thermal process.
- If your primary focus is on processes below 1200°C or requires rapid heating/cooling: Quartz is the superior and more cost-effective choice due to its exceptional thermal shock resistance.
- If your primary focus is on maximum temperature (>1200°C) and chemical stability: Alumina is the necessary choice for its ability to maintain structural and chemical integrity in extreme heat.
Ultimately, understanding the properties of these materials empowers you to select the precise tool required for a controlled and successful outcome.
Summary Table:
| Material | Max Temperature | Key Properties | Best For |
|---|---|---|---|
| Quartz (Fused Silica) | Up to 1200°C | Excellent thermal shock resistance, transparent, chemically inert | Processes below 1200°C, rapid heating/cooling, visual observation |
| Alumina (Al₂O₃) | Up to 1700°C+ | High-temperature stability, mechanical durability, superior chemical inertness | High-temp applications (>1200°C), sensitive processes, advanced materials |
Struggling to find the perfect tubular furnace tube for your unique experimental setup? KINTEK specializes in advanced high-temperature furnace solutions, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With our exceptional R&D and in-house manufacturing, we offer deep customization to precisely match your temperature and material requirements, ensuring purity and success in your lab. Contact us today to discuss how we can enhance your thermal processing with tailored solutions!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















