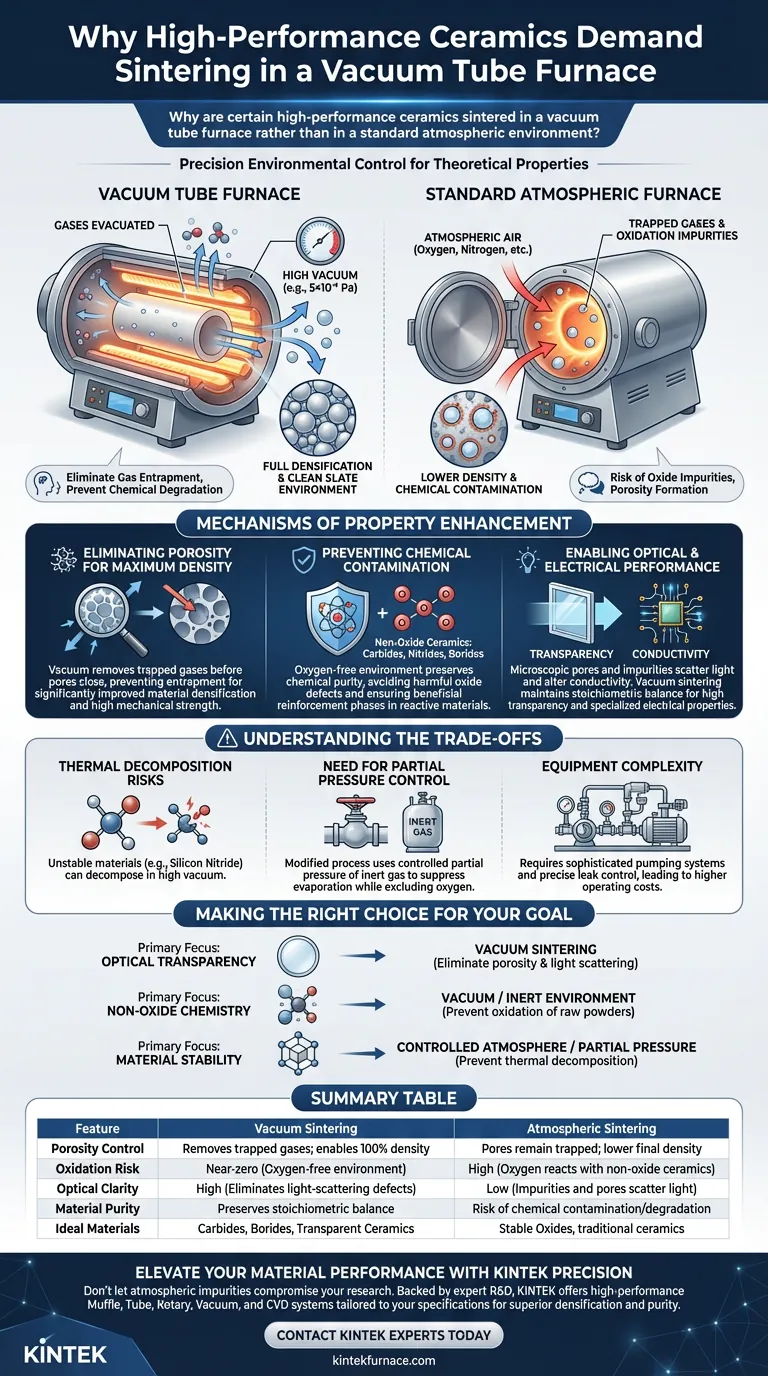
High-performance ceramics require precise environmental control to achieve their theoretical properties. Sintering in a vacuum tube furnace is primarily chosen to eliminate gas entrapment within the material and prevent chemical degradation. Unlike standard atmospheric furnaces, a vacuum environment removes air from pores to ensure full densification and shields reactive materials from oxidation or unwanted nitrification at high temperatures.
Sintering in a vacuum is not merely about heating; it is about creating a "clean slate" environment. By actively evacuating gases, the process prevents the formation of oxide impurities and collapses pores that would otherwise destroy the material's transparency, mechanical strength, and electrical conductivity.

The Mechanisms of Property Enhancement
Eliminating Porosity for Maximum Density
The primary physical benefit of a vacuum environment is the removal of gases trapped in closed pores between material particles. In an atmospheric furnace, pockets of air can become sealed inside the ceramic as it densifies, preventing the material from shrinking fully.
By lowering the pressure, the vacuum effectively pulls these gases out before the pores close. This leads to significantly improved material densification, which is a prerequisite for high mechanical strength.
Preventing Chemical Contamination
Many advanced ceramics, such as carbides, nitrides, and borides, are highly reactive with oxygen at sintering temperatures (often exceeding 1700°C). Heating these materials in standard air causes immediate oxidation, forming impurities that degrade performance.
A vacuum tube furnace provides an oxygen-free environment. This preserves the chemical purity of the raw materials, ensuring that reactions (such as those between silicon and boron carbide) produce beneficial reinforcement phases rather than harmful oxide defects.
Enabling Optical and Electrical Performance
For functional ceramics, purity is directly linked to performance. If a ceramic is intended to be transparent, even microscopic pores or oxide inclusions will scatter light and ruin optical clarity.
Similarly, for electrical or thermoelectric materials like Silicon-Germanium (SiGe) alloys, oxidation alters the material's conductivity. Vacuum sintering maintains the stoichiometric balance required for specialized electrical properties and high transparency.
Understanding the Trade-offs: Volatility and Stability
While vacuum sintering offers superior purity, it introduces thermodynamic challenges that must be managed.
Thermal Decomposition Risks
Not all materials remain stable in a high vacuum at high temperatures. For example, silicon nitride is thermodynamically unstable under these conditions and can decompose into silicon and nitrogen gas.
The Need for Partial Pressure Control
To counter decomposition, the "vacuum" process is often modified to include a controlled partial pressure of inert gas (like high-purity nitrogen or argon). This suppresses the evaporation of volatile elements while still excluding oxygen.
Equipment Complexity
Vacuum tube furnaces are significantly more complex and expensive to operate than atmospheric furnaces. They require sophisticated pumping systems and precise leak control to maintain the necessary pressure levels (e.g., 5×10⁻² Pa) over long cycles.
Making the Right Choice for Your Goal
To determine if vacuum sintering is the correct approach for your application, evaluate your specific material constraints.
- If your primary focus is Optical Transparency: You must use vacuum sintering to fully evacuate trapped gases and eliminate the porosity that causes light scattering.
- If your primary focus is Non-Oxide Chemistry: You require a vacuum or inert environment to prevent the raw ceramic powders (like Silicon or Boron Carbide) from reacting with atmospheric oxygen.
- If your primary focus is Material Stability (e.g., Silicon Nitride): You should use a furnace capable of controlled atmosphere or partial pressure rather than a high vacuum to prevent thermal decomposition.
Ultimate material performance is rarely limited by the raw ingredients, but rather by the purity of the environment in which they are fused.
Summary Table:
| Feature | Vacuum Sintering | Atmospheric Sintering |
|---|---|---|
| Porosity Control | Removes trapped gases; enables 100% density | Pores remain trapped; lower final density |
| Oxidation Risk | Near-zero (Oxygen-free environment) | High (Oxygen reacts with non-oxide ceramics) |
| Optical Clarity | High (Eliminates light-scattering defects) | Low (Impurities and pores scatter light) |
| Material Purity | Preserves stoichiometric balance | Risk of chemical contamination/degradation |
| Ideal Materials | Carbides, Borides, Transparent Ceramics | Stable Oxides, traditional ceramics |
Elevate Your Material Performance with KINTEK Precision
Don’t let atmospheric impurities compromise your research or production quality. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your exact specifications. Whether you are aiming for theoretical density in advanced ceramics or specialized electrical properties, our customizable lab high-temperature furnaces provide the "clean slate" environment your materials require.
Ready to achieve superior densification and purity?
Contact KINTEK Experts Today to find the perfect thermal processing solution for your unique needs.
Visual Guide
References
- Wencke Mohring, Christiane Stephan‐Scherb. High-Temperature Corrosion of High- and Medium-Entropy Alloys CrMnFeCoNi and CrCoNi Exposed to a Multi-Oxidant Atmosphere H2O–O2–SO2. DOI: 10.1007/s44210-023-00026-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the function of an industrial tube furnace during the secondary carbonization of biomass? Achieve Precision.
- What are the standard and customizable options for tube furnaces? Find the Perfect Fit for Your Lab's Needs
- How does a fast Joule-heating device differ from a tubular furnace? Kinetic vs. Thermodynamic Control
- Why is it necessary to maintain a vacuum inside the tube? To Enable Controlled Electron Flow
- What conditions does a continuous flow fixed-bed quartz reactor provide? Master CO Oxidation Testing with Cobalt Oxide
- What role do vacuum tube furnaces play in ceramic and glass manufacturing? Unlock High-Purity, Dense Materials
- What is the function of a Tube Furnace in the preparation of WSe2 thin films? Master Precise Atomic Deposition
- What are the primary benefits of using a split tube furnace? Enhance Lab Efficiency with Unmatched Flexibility



















