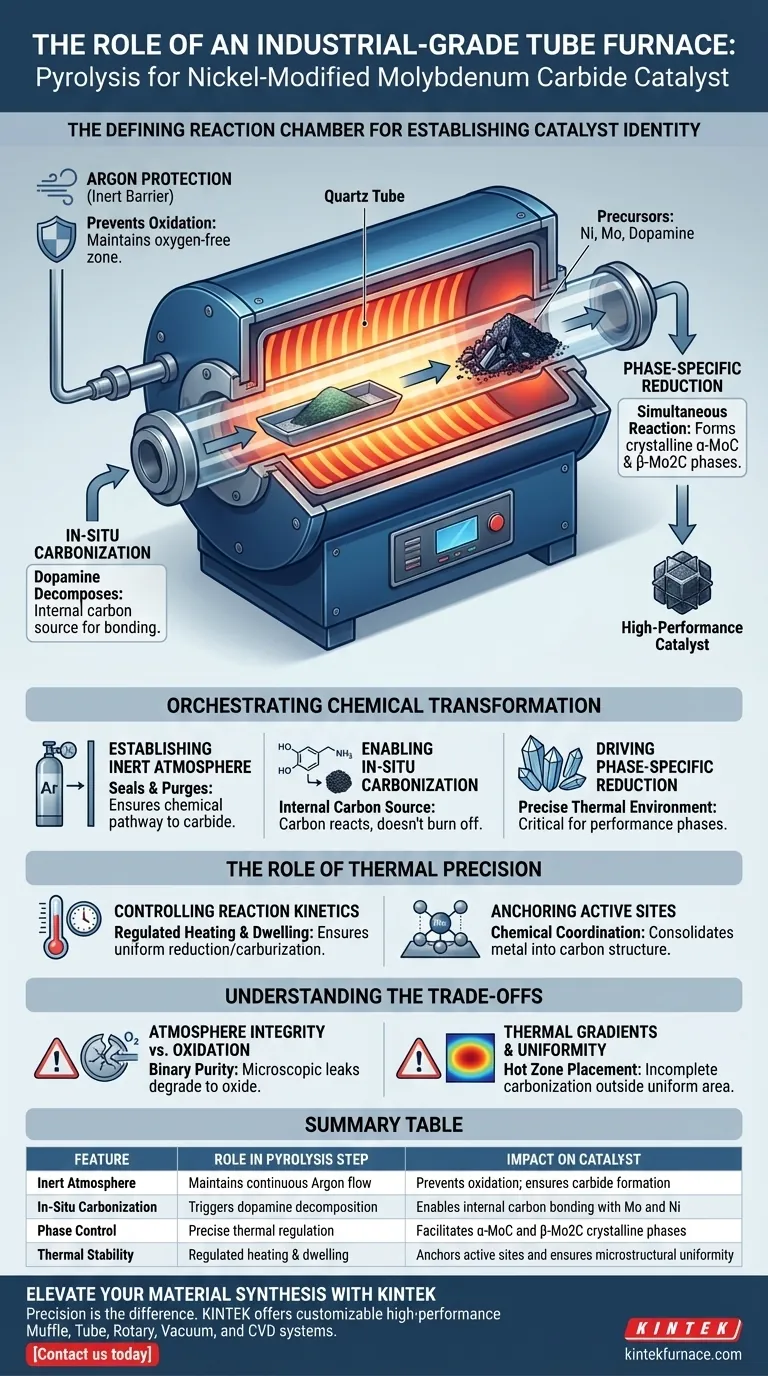
The industrial-grade tube furnace serves as the defining reaction chamber where the chemical identity of the catalyst is established. It provides a sealed, oxygen-free environment—specifically under argon protection—that allows for the precise thermal conversion of precursors. This controlled isolation is the only way to transform raw components into high-performance nickel-modified molybdenum carbide without destructive oxidation.
Core Insight: The tube furnace is not merely a heater; it is a reactor that enforces in-situ carbonization and reduction. By maintaining a strict argon atmosphere, it enables the carbon source (dopamine) to chemically bond with molybdenum and nickel, driving the formation of essential crystalline phases like $\alpha$-MoC and $\beta$-Mo2C.

Orchestrating the Chemical Transformation
The pyrolysis step is a complex physicochemical process where the tube furnace acts as the primary control mechanism. It aligns thermal energy with atmospheric isolation to dictate the final structure of the material.
Establishing the Inert Atmosphere
The primary function of the furnace during this specific synthesis is to maintain argon protection. This creates an inert barrier that prevents atmospheric oxygen from interfering with the reaction.
Without this oxygen-free zone, the precursors would simply oxidize rather than carbonize. The furnace's ability to seal and purge the environment ensures that the chemical pathway remains focused on carbide formation.
Enabling In-Situ Carbonization
Inside the furnace, the high temperatures trigger the decomposition of dopamine, which serves as the internal carbon source.
Because the environment is controlled, the carbon released from dopamine does not burn off; instead, it remains available to react. This process is known as in-situ carbonization, where the carbon source is derived directly from the precursor mixture during heating.
Driving Phase-Specific Reduction
The furnace facilitates a simultaneous reduction reaction between the carbon, molybdenum, and nickel components.
This reaction is highly sensitive to temperature and results in the generation of specific crystalline phases. The precise thermal environment allows for the successful synthesis of $\alpha$-MoC (alpha-molybdenum carbide) and $\beta$-Mo2C (beta-molybdenum carbide), which are critical for the catalyst's performance.
The Role of Thermal Precision
Beyond atmosphere, the tube furnace provides the thermal stability required to ensure the catalyst forms a consistent microstructure.
Controlling Reaction Kinetics
The furnace ensures that the heating rate and dwelling time are strictly regulated.
If the temperature fluctuates, the reaction between the metal ions and the carbon source becomes unpredictable. Precise control ensures that the molybdenum and nickel are reduced and carburized uniformly across the entire batch.
Anchoring Active Sites
The thermal treatment consolidates the metal components, ensuring they are integrated into the carbon structure rather than loosely attached.
This high-temperature processing facilitates the chemical coordination between the metals and the support. It creates a robust material where the active catalytic sites are stable and chemically bonded to the substrate.
Understanding the Trade-offs
While the tube furnace is essential for synthesis, there are operational constraints and risks that must be managed to ensure success.
Atmosphere Integrity vs. Oxidation
The most critical failure point is the seal of the tube furnace. Even a microscopic leak allows oxygen ingress, which will instantly degrade the molybdenum carbide into molybdenum oxide.
Atmosphere purity is binary: it is either maintained perfectly, or the batch is compromised. There is very little margin for error regarding gas flow rates and seal integrity.
Thermal Gradients and Uniformity
While the furnace controls temperature, thermal gradients can exist along the length of the tube.
If the precursors are placed outside the "hot zone" (the area of uniform temperature), the carbonization may be incomplete. This leads to a heterogeneous product where some portions lack the required $\alpha$-MoC or $\beta$-Mo2C crystalline phases.
Making the Right Choice for Your Goal
To optimize the preparation of nickel-modified molybdenum carbide, you must align your furnace operation with your specific synthesis targets.
- If your primary focus is Phase Purity: Ensure your argon flow is continuous and the system is thoroughly purged before heating to prevent any oxide formation.
- If your primary focus is Microstructure Consistency: Calibrate the "hot zone" of your furnace and place precursors only in the region where the temperature deviation is minimal (< +/- 5°C).
- If your primary focus is Scalability: Prioritize furnace designs that maintain uniform gas flow dynamics over larger volumes to ensure the dopamine-derived carbon reacts evenly with the metal bed.
Success in this synthesis relies not just on reaching high temperatures, but on maintaining the absolute purity of the reducing environment throughout the entire thermal cycle.
Summary Table:
| Feature | Role in Pyrolysis Step | Impact on Catalyst |
|---|---|---|
| Inert Atmosphere | Maintains continuous Argon flow | Prevents oxidation; ensures carbide formation |
| In-Situ Carbonization | Triggers dopamine decomposition | Enables internal carbon bonding with Mo and Ni |
| Phase Control | Precise thermal regulation | Facilitates $\alpha$-MoC and $\beta$-Mo2C crystalline phases |
| Thermal Stability | Regulated heating & dwelling | Anchors active sites and ensures microstructural uniformity |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a failed batch and a high-performance catalyst. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous demands of your laboratory or industrial application.
Whether you are synthesizing nickel-modified molybdenum carbide or developing next-generation materials, our high-temperature furnaces provide the atmospheric integrity and thermal uniformity you need to succeed. Contact us today to discuss your unique project requirements and discover how KINTEK can optimize your thermal processing workflow.
Visual Guide
References
- Ying Yang, Kunyu Xu. Controllable synthesis of transition metal-modified molybdenum carbide crystalline phases and its application on hydrodeoxygenation of phenol. DOI: 10.1051/e3sconf/202562501016
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is high-precision temperature control in a tube furnace critical for rhenium catalysts? Ensure Optimal Alumina Calcination
- What are the different types of tube furnaces available? Find the Perfect Fit for Your Lab's Needs
- What environmental conditions does a high-temperature tube furnace simulate for corrosion? Replicate Boiler Realities
- Why is a high-temperature tube furnace required for the secondary activation of KBC? Achieve Precision Pore Structure
- How does a tube furnace facilitate the carbonization of ZIFs while preventing oxidation? Expert Insights
- Why is a high-precision programmable tube furnace required for N-doped TiO2@C composites? Expert Thermal Solutions
- Why is controlling the residence time within a tube furnace critical for the synthesis of amorphous NiFe2O4 catalysts?
- What is a tube furnace and what are its applications? Unlock Precision Heating for Advanced Materials



















