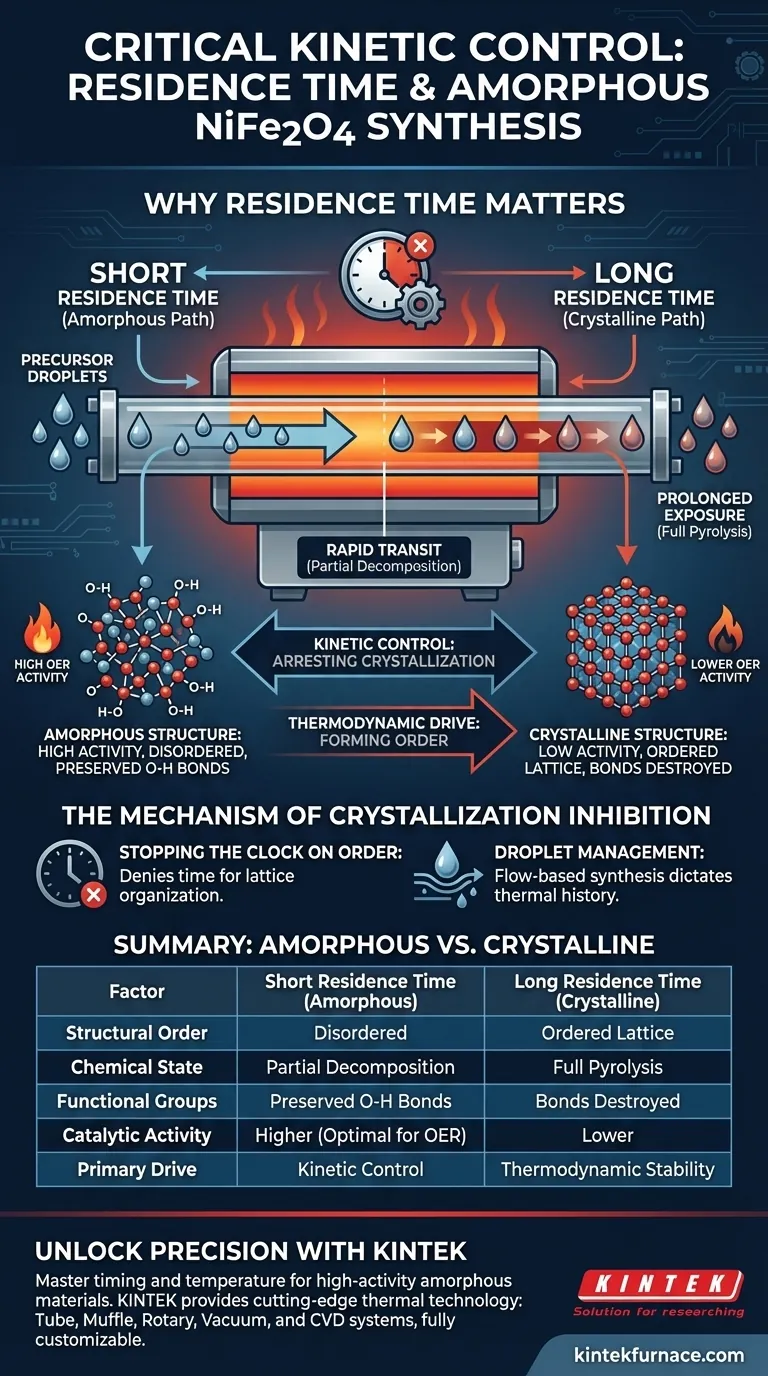
Controlling residence time is the single most critical variable for arresting the thermodynamic drive toward crystallization. In the synthesis of amorphous NiFe2O4, a short residence time restricts the duration precursor droplets spend in the heated zone, physically preventing the formation of a long-range ordered lattice.
The Core Takeaway Achieving an amorphous structure requires precise kinetic control to interrupt the transition from precursor to crystal. By limiting residence time, you ensure the material undergoes only partial decomposition, preserving the disordered structures and chemical bonds essential for high electrocatalytic activity.

The Mechanism of Crystallization Inhibition
Stopping the Clock on Order
Crystallization is a time-dependent process that requires thermal energy to arrange atoms into a structured lattice.
By shortening the residence time, you deny the material the necessary window to organize. The precursor droplets move through the heated zone too quickly for long-range order to establish itself.
Droplet Management
The primary reference highlights that this process relies specifically on managing the duration of droplets within the furnace.
This implies a flow-based synthesis method (such as spray pyrolysis) where the velocity of the carrier gas directly dictates the thermal history of the particle.
Chemical Transformation and Structure
Partial Decomposition vs. Full Pyrolysis
Standard thermal treatments typically aim for full pyrolysis, where metal nitrates are completely broken down into stable metal oxides.
However, for amorphous NiFe2O4, the goal is partial decomposition. Short residence times stop the chemical reaction midway, preventing the complete conversion that leads to rigid crystalline phases.
Preserving Critical Bonds
The incomplete decomposition process has a specific chemical benefit: it preserves O-H bonds.
These bonds would likely be destroyed during a prolonged, high-temperature heat treatment. Their presence, along with the disordered atomic structure, is directly linked to enhanced performance in the oxygen evolution reaction (OER).
Understanding the Trade-offs
The Precision Window
While shorter is generally better for amorphous synthesis, there is a lower limit to residence time.
If the time is too short, the precursor droplets may not decompose sufficiently to form the active catalyst species at all. The process requires a "Goldilocks" zone—long enough to initiate decomposition of the metal nitrates, but short enough to halt the process before crystallization occurs.
Stability vs. Activity
Amorphous materials often trade thermodynamic stability for catalytic activity.
A crystalline lattice is stable but often less active. By choosing a short residence time, you are prioritizing the high activity derived from defects and disordered sites over the long-term structural stability typical of fully crystalline materials.
Making the Right Choice for Your Goal
To optimize your synthesis parameters, evaluate your specific performance targets:
- If your primary focus is maximizing catalytic activity: Prioritize high flow rates to minimize residence time, ensuring the material remains amorphous and retains O-H bonds.
- If your primary focus is lattice stability: Extend the residence time to allow for full pyrolysis and the development of long-range crystalline order, acknowledging this may reduce OER performance.
Summary: The power of the amorphous NiFe2O4 catalyst lies in its disorder, which is engineered strictly by denying the material the time it needs to crystallize.
Summary Table:
| Factor | Short Residence Time (Amorphous) | Long Residence Time (Crystalline) |
|---|---|---|
| Structural Order | Disordered/Amorphous | Long-range Ordered Lattice |
| Chemical State | Partial Decomposition | Full Pyrolysis |
| Functional Groups | Preserved O-H Bonds | Bonds Destroyed |
| Catalytic Activity | Higher (Optimal for OER) | Lower |
| Primary Drive | Kinetic Control | Thermodynamic Stability |
Unlock Precision in Your Catalyst Synthesis
Precision timing and temperature control are the keys to arresting crystallization and engineering high-activity amorphous materials. KINTEK provides the cutting-edge thermal technology required to master these variables. Backed by expert R&D and precision manufacturing, we offer a comprehensive range of Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory and production needs.
Ready to optimize your synthesis parameters? Contact KINTEK today to discuss how our high-temperature furnace solutions can enhance your research and manufacturing efficiency.
Visual Guide
References
- Jan Witte, Thomas Turek. Efficient Anion Exchange Membrane Water Electrolysis on Amorphous Spray‐Pyrolyzed NiFe<sub>2</sub>O<sub>4</sub>. DOI: 10.1002/celc.202500226
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the main industrial applications of rotary tube furnaces? Boost Efficiency in Metallurgy and Materials Processing
- What are some primary applications of the 70mm tube furnace? Unlock Precision in Materials Research
- What are the types of Tube Furnaces based on orientation? Horizontal vs. Vertical for Optimal Thermal Processing
- What are the benefits of using tube furnaces in industrial processes? Achieve Precision and Control for Your Lab
- Why is a tube furnace with nitrogen flow necessary for BaFe2-xCoxFe16O27 ceramics? Master Iron Valence Engineering
- Why is a tantalum tube encapsulated in a vacuum quartz tube? Prevent Oxidation & Embrittlement in High-Temp Calcination
- Why Use a Reducing Gas in Tube Furnace Thermal Treatment? Unlock Pure Metallic Phases and Defects
- What core experimental conditions does a horizontal high-temperature tube furnace provide for measuring chromium volatilization?



















