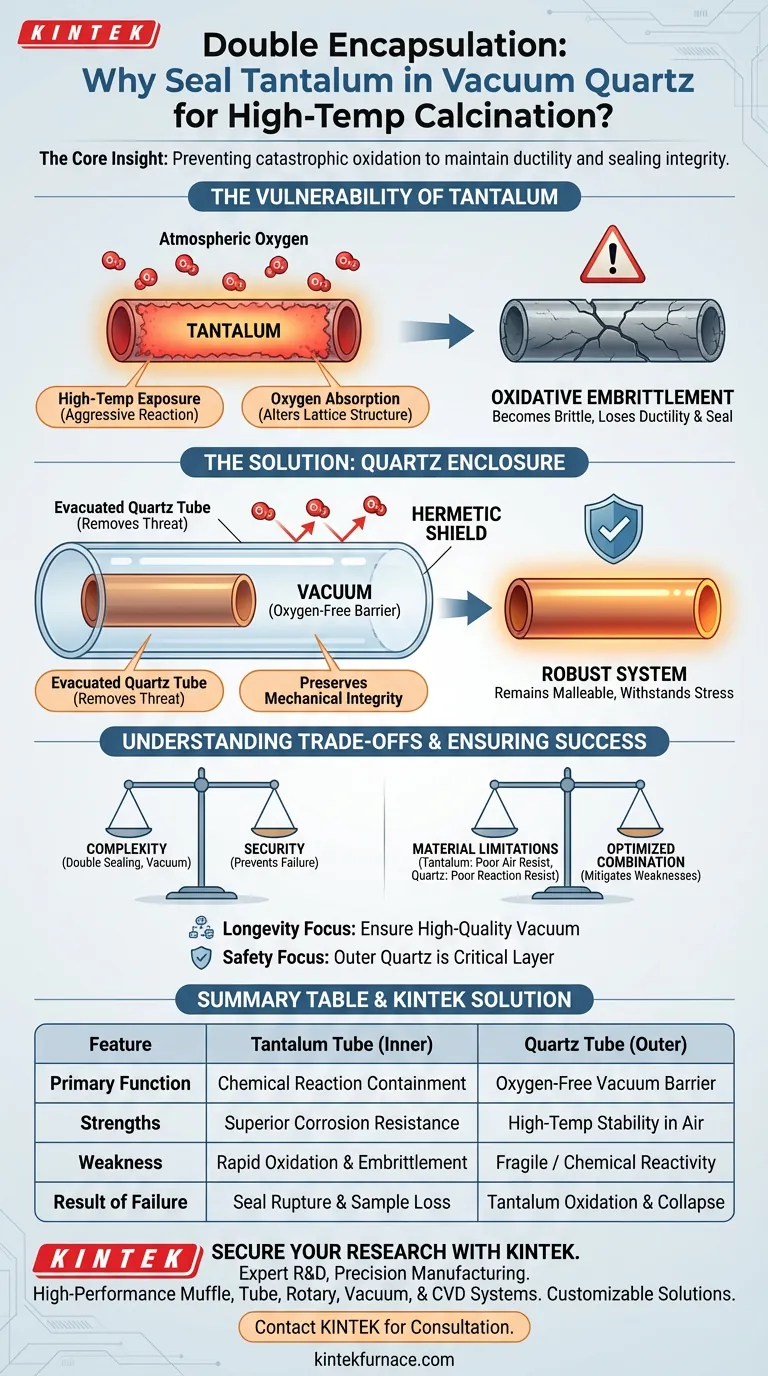
The primary purpose of encapsulating a sealed tantalum tube within a vacuum quartz tube is to prevent catastrophic oxidation. While tantalum is renowned for its general corrosion resistance, it possesses a critical vulnerability when exposed to atmospheric oxygen at high temperatures. The outer quartz tube, being evacuated of air, acts as a hermetic shield that prevents oxygen from reaching the tantalum surface, thereby preserving the metal's mechanical properties.
The Core Insight High-temperature calcination exposes tantalum to a specific failure mode: oxidative embrittlement. By isolating the tantalum tube inside an evacuated quartz vessel, you serve the deep need of the experiment—maintaining the ductility and sealing integrity of the reaction vessel during prolonged heating.
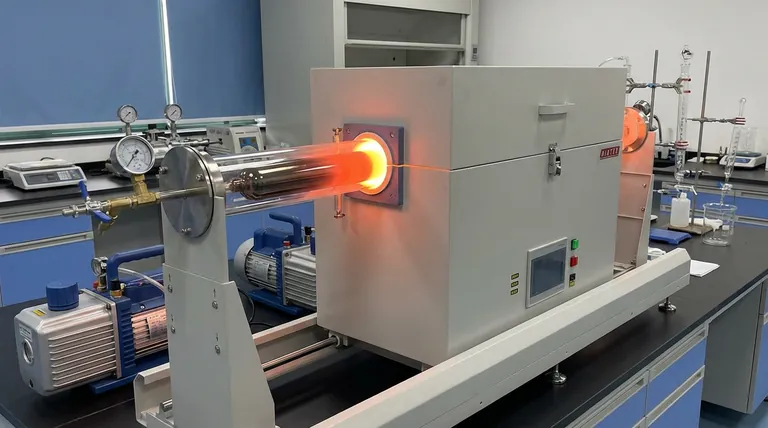
The Vulnerability of Tantalum
Corrosion Resistance vs. High-Temperature Stability
It is a common misconception that because tantalum is corrosion-resistant, it is impervious to all environments. While it resists chemical attack well, tantalum reacts aggressively with oxygen when heated to calcination temperatures.
The Risk of Embrittlement
When tantalum oxidizes in the atmosphere, it does not merely rust on the surface. The metal absorbs oxygen, which fundamentally alters its internal lattice structure. This process causes the tantalum to become extremely brittle, causing it to lose the ductility necessary to maintain a high-pressure seal.
The Role of the Quartz Enclosure
Creating an Oxygen-Free Barrier
The quartz tube serves as the primary environmental control mechanism. By evacuating the quartz tube (creating a vacuum) before sealing it, you remove the source of the threat: atmospheric oxygen. This ensures the tantalum remains in an inert environment, regardless of the temperature outside the quartz.
Preserving Mechanical Integrity
The ultimate goal of this setup is to maintain the mechanical strength of the inner tube. If the tantalum were to become brittle, thermal expansion or internal pressure from the experiment could cause the tube to crack or shatter. The secondary quartz layer ensures the tantalum remains malleable enough to withstand the physical stresses of the experiment.
Understanding the Trade-offs
Complexity vs. Security
The decision to use a double-encapsulation method adds complexity to the experimental setup. It requires precise sealing of two distinct layers and the capability to create a vacuum. However, this complexity is the unavoidable "cost" of using tantalum at high temperatures; without it, the equipment is virtually guaranteed to fail.
Material Limitations
This setup highlights that no single material is perfect for all conditions. Tantalum is excellent for containing the reaction but poor at resisting external air at heat. Quartz is excellent at resisting air and heat but may not be suitable for the internal reaction; combining them mitigates the specific weaknesses of both.
Ensuring Experimental Success
To apply this to your specific project, consider the following principles:
- If your primary focus is Equipment Longevity: Ensure the vacuum in the quartz tube is high quality; even trace oxygen can degrade the tantalum over prolonged calcination.
- If your primary focus is Safety: Treat the outer quartz tube as a critical containment layer; if it compromises, the inner tantalum tube is likely to fail shortly after due to rapid oxidation.
By shielding the tantalum from its primary weakness—oxygen—you transform a fragile setup into a robust system capable of enduring prolonged high-temperature operations.
Summary Table:
| Feature | Tantalum Tube (Inner) | Quartz Tube (Outer) |
|---|---|---|
| Primary Function | Chemical reaction containment | Oxygen-free vacuum barrier |
| Strengths | Superior corrosion resistance | High-temp stability in air |
| Weakness | Rapid oxidation & embrittlement | Fragile / Chemical reactivity |
| Result of Failure | Seal rupture and sample loss | Tantalum oxidation and structural collapse |
Secure Your High-Temperature Research with KINTEK
Don't let oxidative failure compromise your results. At KINTEK, we understand the complex material science behind high-temperature calcination. Backed by expert R&D and precision manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet your most rigorous lab requirements. Whether you need standard equipment or a fully customizable system for specialized double-encapsulation workflows, our team is ready to support your unique research needs.
Protect your experiments and enhance lab efficiency—Contact KINTEK today for a consultation!
Visual Guide
References
- Investigation of a Ternary Zintl Phase KBaBi: Synthesis, Crystal Structure, and Preliminary Transport Properties. DOI: 10.1002/zaac.202500064
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What distinguishes a compact tube furnace from other types? Ideal for Small-Scale Lab Precision
- What physical conditions do high-temperature tube furnaces provide for flue gas kinetics? Precision Thermal Simulation
- What is the role of a tubular furnace in the conversion of coffee ground powder into biochar? Master Precise Pyrolysis
- What conditions does a laboratory tube furnace provide for PtS/Ti3C2Tx preparation? Master 300°C Thermal Decomposition
- What role does a laboratory tube furnace play in STO thin film annealing? Unlock Neuromorphic Potential
- What is the temperature of a tube furnace? Selecting the Right High-Temp Solution for Your Lab
- What role does a tube furnace play in the conversion of sludge into biochar? Master Precise Thermal Pyrolysis
- How does a horizontal tube furnace ensure experimental safety and accuracy during the thermal dehydrogenation of Ca(AlH4)2?



















