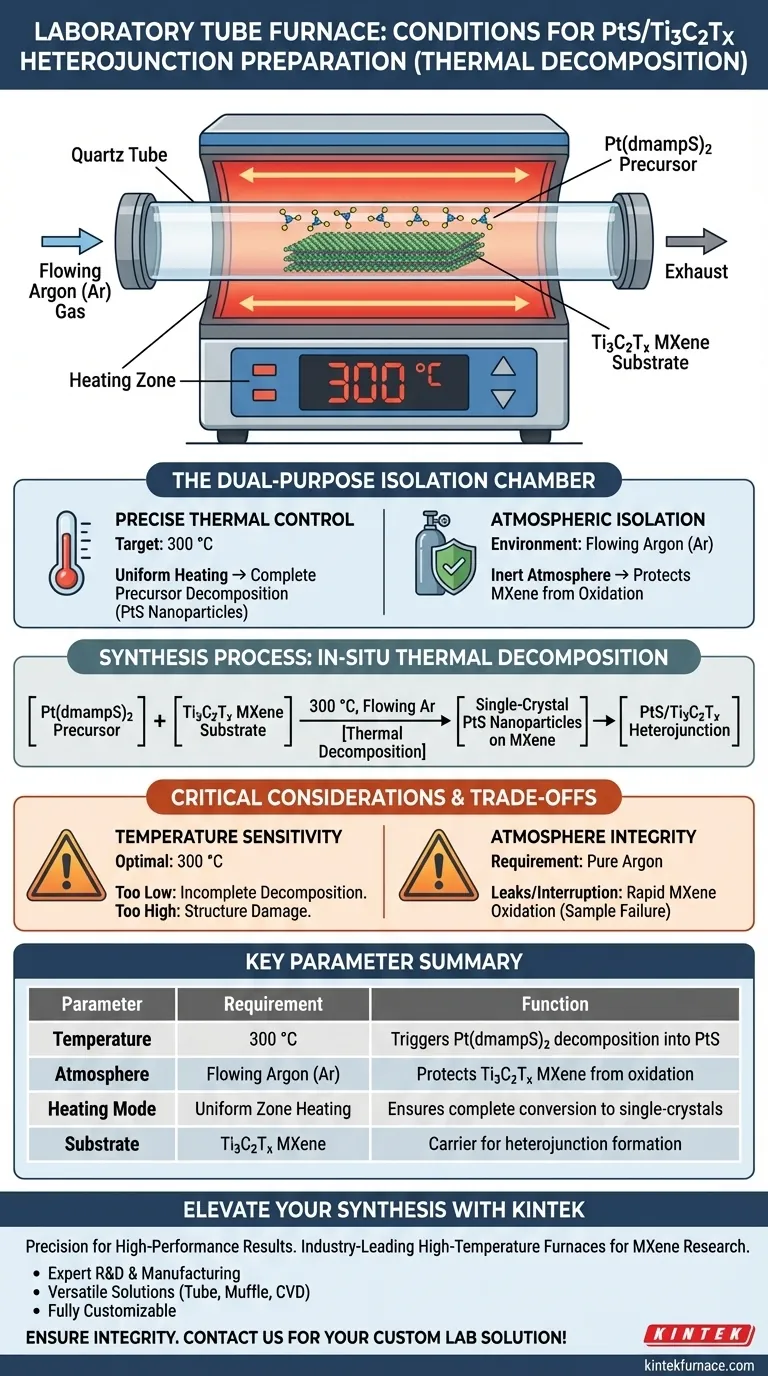
A laboratory tube furnace establishes the critical environment for synthesizing PtS/Ti3C2Tx heterojunctions by maintaining a precise temperature of 300 °C under a continuous flow of Argon (Ar) gas. This specific combination allows for the in-situ thermal decomposition of the Pt(dmampS)2 precursor directly onto the MXene surface without degrading the substrate.
The tube furnace functions as a dual-purpose isolation chamber: it provides the thermal energy required to decompose precursors into single-crystal nanoparticles while simultaneously maintaining an inert atmosphere that strictly protects the sensitive MXene carrier from high-temperature oxidation.

Precise Thermal Control
Targeting the Decomposition Threshold
The synthesis relies on holding the reaction environment at exactly 300 °C. This specific temperature is calibrated to trigger the conversion of the Pt(dmampS)2 precursor.
Ensuring Complete Conversion
The laboratory tube furnace provides uniform heating throughout the reaction zone. This consistency guarantees the complete decomposition of the precursor, resulting in the formation of single-crystal platinum monosulfide (PtS) nanoparticles.
Atmospheric Isolation
The Role of Inert Gas
The process must occur under a flowing Argon (Ar) atmosphere. This continuous flow flushes out ambient air and creates a stable, inert environment within the tube.
Preventing Substrate Degradation
The Ti3C2Tx MXene carrier is highly susceptible to oxidation when exposed to heat. By excluding environmental contaminants and oxygen, the Ar atmosphere ensures the MXene retains its structural integrity during the formation of the heterojunction.
Critical Considerations and Trade-offs
Temperature Sensitivity
While 300 °C is the optimal setpoint, deviation can compromise the material. Temperatures that are too low may result in incomplete precursor decomposition, while excessive heat could damage the heterojunction structure even within an inert atmosphere.
Atmosphere Integrity
The success of this method is entirely dependent on the purity of the Argon environment. Any leak in the furnace seals or interruption in gas flow will lead to rapid oxidation of the MXene, rendering the sample unusable.
Optimizing Your Synthesis Strategy
To ensure the successful preparation of PtS/Ti3C2Tx heterojunctions, focus on the strict regulation of heat and gas flow.
- If your primary focus is Material Purity: Ensure the Argon flow is fully established to purge contaminants before the heating ramp begins.
- If your primary focus is Crystallinity: Maintain the 300 °C temperature with high precision to facilitate the growth of high-quality single-crystal PtS nanoparticles.
Precise control over these environmental variables acts as the safeguard for creating high-performance heterojunctions.
Summary Table:
| Key Parameter | Requirement | Function in Synthesis |
|---|---|---|
| Temperature | 300 °C | Triggers Pt(dmampS)2 decomposition into PtS |
| Atmosphere | Flowing Argon (Ar) | Protects Ti3C2Tx MXene from high-temp oxidation |
| Heating Mode | Uniform Zone Heating | Ensures complete conversion to single-crystal nanoparticles |
| Substrate | Ti3C2Tx MXene | Serves as the carrier for heterojunction formation |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a high-performance heterojunction and a failed sample. KINTEK provides industry-leading laboratory high-temperature furnaces designed for the rigorous demands of MXene research and thermal decomposition.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our systems offer the ultra-stable thermal zones and gas-tight integrity required for sensitive Ar-flow processes.
- Versatile Solutions: From standard Tube and Muffle furnaces to Rotary, Vacuum, and CVD systems.
- Fully Customizable: We tailor our equipment to meet your unique temperature profiles and atmospheric needs.
Ensure the integrity of your PtS/Ti3C2Tx heterojunctions with KINTEK’s reliable heating technology. Contact us today to find your custom lab solution!
Visual Guide
References
- Young-Hee Park, Jongsun Lim. Direct Growth of Platinum Monosulfide Nanoparticles on MXene via Single‐Source Precursor for Enhanced Hydrogen Evolution Reaction. DOI: 10.1002/smsc.202500407
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is a laboratory tube furnace? Master Precision Heating and Atmosphere Control
- Why is a high-temperature tube furnace utilized for the calcination of nano-zinc oxide? Master Microstructure Control
- What types of heating mechanisms are employed in drop tube furnaces? Choose Between Resistive and Induction Heating
- What is the function of a high-temperature tube furnace in the annealing treatment of ZnIn electrodes?
- Why is a high-temperature tube furnace utilized for the pyrolysis of Sr2TiO4 precursor powders? Achieving High Purity
- How did the tube furnace originate and where is it commonly used today? Discover Its Evolution and Modern Applications
- What role does an atmosphere-controlled vacuum tube furnace play in sintering? Mastering Porous Stainless Steel
- What are the advantages of microwave heating tube furnaces? Achieve Fast, Uniform, and Efficient Material Processing



















